People's Education Edition Physics for Grade 8, Volume 2
People's Education Edition Ninth Grade Physics Complete Book
Shanghai Science Edition Ninth Grade Physics
People's Education Edition Physics for Grade 8, Volume 1
Beijing Normal University eighth grade physics volume one
Shanghai Science Edition 8th Grade Physics
Lu Jiao Edition Ninth Grade Physics Volume 1
Beijing Normal University Ninth Grade Physics Volume 1
Guangdong and Shanghai Edition Ninth Grade Physics Volume 1
Lu Jiao Edition Ninth Grade Physics Volume 2
Lu Ke Edition High School Physics Compulsory Course One
People's Education Press High School Physics Compulsory Course II
Beijing Normal University Ninth Grade Physics Volume 2
Lu Jiao Edition Eighth Grade Physics Volume 2
Guangdong and Shanghai version of eighth grade physics volume 2
Lu Jiao edition eighth grade physics volume 1

| Category | Format | Size |
|---|---|---|
| People's Education Edition Ninth Grade Physics Complete Book | pptx | 6 MB |
Description
"Thermal Motion of Molecules" Internal Energy PPT Courseware 3
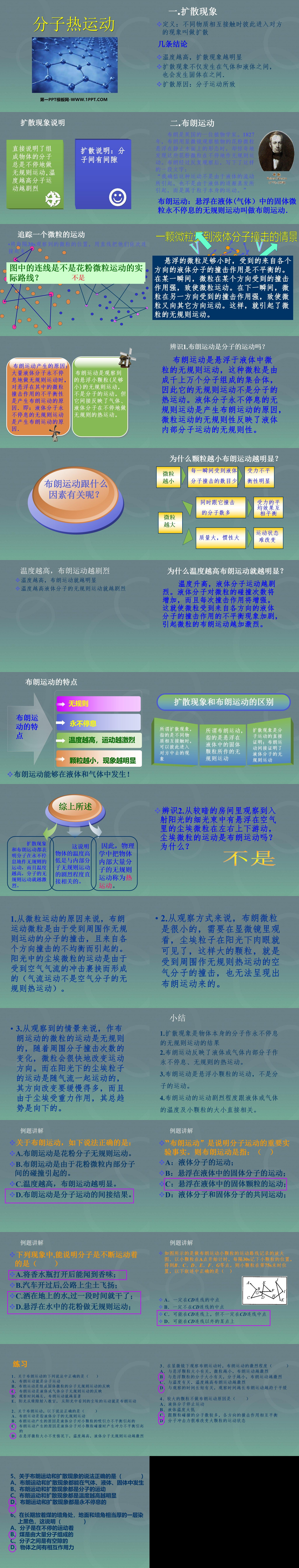
1. Diffusion phenomenon
Definition: The phenomenon of different substances entering each other when they come into contact is called diffusion
Several conclusions
The higher the temperature, the more obvious the diffusion phenomenon
Diffusion occurs not only between gases and liquids, but also between solids.
Cause of diffusion: due to molecular movement
Explanation of diffusion phenomenon
It directly shows that the molecules that make up an object are always moving irregularly. The higher the temperature, the more violent the movement of the molecules.
Diffusion description: There are gaps between molecules
The cause of Brownian motion: When a large number of liquid molecules are constantly moving irregularly, the unbalanced impact on the particles suspended in them is the cause of Brownian motion. That is: the never-ending irregular motion of liquid molecules is the cause of Brownian motion.
Brownian motion is the observed random motion of suspended small particles (small enough), not the motion of molecules. But it indirectly reflects that gas and liquid molecules are constantly making irregular thermal motions.
Why does Brownian motion become more obvious the higher the temperature?
As the temperature increases, the movement of liquid molecules becomes more intense. The number of collisions between liquid molecules and particles will increase, and the effect of each collision will be stronger. This intensifies the imbalance of the particles being impacted by liquid molecules from all directions, causing the Brownian motion of the particles to become more intense.
summary
1. The phenomenon of diffusion is the result of the endless and irregular motion of the molecules of the object itself.
2. Brownian motion reflects the never-ending and irregular thermal motion of molecules inside a liquid or gas.
3. Brownian motion is the motion of suspended small particles, not the motion of molecules.
4. The intensity of Brownian motion is directly related to the temperature of the liquid or gas and the size of the small particles.
Regarding Brownian motion, the following statement is correct:
A. Brownian motion is the irregular movement of pollen molecules.
B. Brownian motion is caused by collisions between molecules within pollen particles.
C. The higher the temperature, the more obvious the Brownian motion.
D. Brownian motion is an indirect result of molecular motion.
"Brownian motion" is an important experimental fact explaining the motion of molecules. Then Brownian motion refers to: ( )
A: Movement of liquid molecules;
B: Movement of solid molecules suspended in liquid;
C: Movement of solid particles suspended in liquid;
D: Common motion of liquid molecules and solid molecules;
practise
1. Which of the following statements about Brownian motion is correct ( )
A. Brownian motion is molecular motion
B. Brownian motion is a reflection of the irregular motion of molecules that make up solid particles.
C. Brownian motion is a reflection of the irregular motion of liquid or gas molecules
D. The longer the observation time, the more significant the Brownian motion
E. Sunlight shines into the classroom from the gap, and the movement of dust seen in the sun is Brownian motion.
2. Regarding Brownian motion, which of the following statements is correct ( )
A. Brownian motion refers to the irregular movement of liquid molecules
B. Brownian motion is caused by the unbalanced attraction of liquid molecules to small particles.
C. Brownian motion is caused by the unbalanced impulse produced when liquid molecules collide with small particles.
D. When the size of suspended particles remains unchanged, the higher the temperature, the more intense the irregular movement of liquid molecules.
3. When observing Brownian motion under a microscope, the intensity of Brownian motion ( )
A. It is related to the size of suspended particles. The smaller the particles, the more intense the Brownian motion.
B. It is related to the molecular size of suspended particles. The smaller the molecules, the more intense the Brownian motion.
C. It is related to temperature. The higher the temperature, the more intense the Brownian motion is.
D. It is related to the length of observation. The longer the observation time, the flatter the Brownian motion becomes.
4. The reason why larger particles do not undergo Brownian motion is ( )
A. Liquid molecules stop moving
B. The liquid temperature is too low
C. There are more molecules colliding with the particles, and the collisions in all directions balance each other.
D. The molecular impact force is difficult to change the motion state of large particles.
Keywords: Internal energy teaching courseware, molecular thermal motion teaching courseware, New People's Education Edition ninth-grade physics PPT courseware, ninth-grade physics slide courseware download, internal energy PPT courseware download, molecular thermal motion PPT courseware download, .ppt Format
For more information about the "Internal Energy of Molecular Thermal Movement" PPT courseware, please click the "Internal Energy of Molecular Thermal Movement ppt ppt" tab.
"Thermal Motion of Molecular" Heat and Energy PPT Courseware 4:
"Thermal Motion of Molecular" Heat and Energy PPT Courseware 4 Do you understand the scale of matter? Light-years: The diameter of the Milky Way is approximately 100,000 light-years. Kilometers: China has a vast territory, about 5,200 km from east to west and 5,500 km from north to south. Meter: Yao Ming’s height is 2.26 m. mm: ..
"Thermal Motion of Molecular" Heat and Energy PPT Courseware 3:
"Thermal Motion of Molecular" Heat and Energy PPT Courseware 3 1. The composition of matter: *The molecules are very small, with a diameter of about 10-10m *The number of molecules is huge. Normally, there are about 2.71019 molecules in 1cm3 of air. If the speed of the number of people can To reach 10 billion per second, it takes...
"Thermal Motion of Molecular" Heat and Energy PPT Courseware 2:
"Thermal Motion of Molecular" Heat and Energy PPT Courseware 2 Substances are composed of molecules. Substances have different _______ when they are in different states. Most substances change volume when they change from liquid to solid. When the state of a substance changes, the volume changes. The changes are mainly due to the composition...
File Info
Update Time: 2024-08-18
This template belongs to Physics courseware People's Education Edition Ninth Grade Physics Complete Book industry PPT template
"Thermal Motion of Molecules" Internal Energy PPT Courseware 3 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Thermal Motion of Molecules" Internal Energy PPT Courseware 3 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Thermal Motion of Molecules" Internal Energy PPT Courseware 3, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview