People's Education Edition Physics for Grade 8, Volume 2
People's Education Edition Physics for Grade 8, Volume 1
People's Education Edition Ninth Grade Physics Complete Book
Shanghai Science Edition Ninth Grade Physics
Shanghai Science Edition 8th Grade Physics
Beijing Normal University eighth grade physics volume one
Lu Jiao Edition Ninth Grade Physics Volume 2
Beijing Normal University Ninth Grade Physics Volume 1
Lu Ke Edition High School Physics Compulsory Course One
Lu Jiao Edition Ninth Grade Physics Volume 1
People's Education Press High School Physics Compulsory Course II
Guangdong and Shanghai Edition Ninth Grade Physics Volume 1
Beijing Normal University Ninth Grade Physics Volume 2
Lu Jiao Edition Eighth Grade Physics Volume 2
Lu Jiao edition eighth grade physics volume 1
Guangdong and Shanghai Edition Ninth Grade Physics Volume 2

| Category | Format | Size |
|---|---|---|
| People's Education Edition Physics for Grade 8, Volume 1 | pptx | 6 MB |
Description
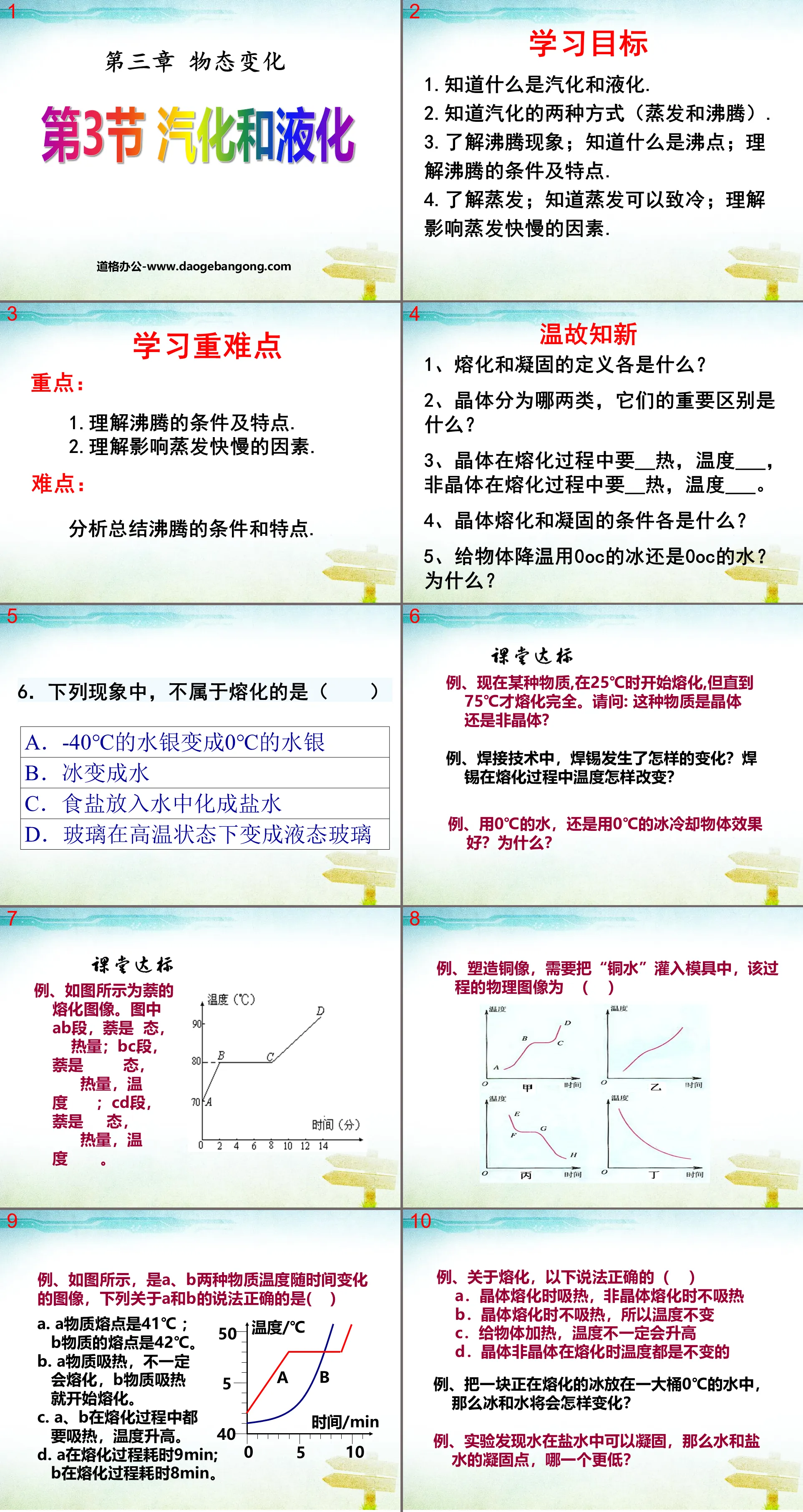
"Vaporization and Liquefaction" PPT Courseware on Changes in State of Matter 6
learning target
1. Know what vaporization and liquefaction are.
2. Know the two ways of vaporization (evaporation and boiling).
3. Understand the phenomenon of boiling; know what boiling point is; understand the conditions and characteristics of boiling.
4. Understand evaporation; know that evaporation can cause cooling; understand the factors that affect the speed of evaporation.
Learn important and difficult points
Key points: 1. Understand the conditions and characteristics of boiling.
2. Understand the factors that affect the speed of evaporation.
Difficulty: Analyze and summarize the conditions and characteristics of boiling.
Review the past and learn the new
1. What are the definitions of melting and solidification?
2. What are the two types of crystals, and what are their important differences?
3. Crystals require __ heat and temperature ___ during the melting process, while amorphous materials require __ heat and temperature ___ during the melting process.
4. What are the conditions for crystal melting and solidification?
5. Should we use 0oC ice or 0oC water to cool objects? Why?
Classroom meets standards
For example, a certain substance starts to melt at 25℃, but does not completely melt until 75℃. I would like to ask: Is this substance crystalline or amorphous?
For example, in welding technology, how has solder changed? How does the temperature of solder change during the melting process?
For example, is it better to use 0℃ water or 0℃ ice to cool an object? Why?
For example, regarding melting, which of the following statements is correct ( )
A. Crystals absorb heat when melting, while amorphous crystals do not absorb heat when melting.
B. The crystal does not absorb heat when melting, so the temperature does not change
C. Heating an object does not necessarily increase its temperature
D. The temperature of crystalline and amorphous crystals does not change when melting.
1. Vaporization and liquefaction
Self-learning
Read the content on pages 58-61 of the textbook (boiling and evaporation) and complete the following pre-exercise questions.
1. Boiling refers to the ____ phenomenon in which liquid ____ and ____ occur at the same time.
2. In the process of water boiling, the temperature ___, but it must be constantly ___. The boiling point refers to _____.
3. Evaporation refers to ______, and evaporation only occurs in _____.
4. ___ and ____ are two ways of vaporization.
5. After rubbing alcohol on the back of your hand, the area where the alcohol is applied will feel cold. Why?
2. Explore: Boiling Characteristics of Water
Ask a question:
1. What are the characteristics of the temperature changes of water before and after boiling?
The temperature rises before boiling and remains constant after boiling
2. If the water continues to be heated after it boils, will the temperature get higher and higher?
3. What will happen if the alcohol lamp is removed after the water boils? Will it stop boiling immediately? What does it mean?
Will not stop boiling immediately, will stop boiling after a while
Analysis and Demonstration
1. What are the conditions for boiling? Reach boiling point, continue heating
2. What are the characteristics of boiling? Absorb heat but the temperature remains unchanged
3. How does the size of bubbles change before boiling and during boiling? Before boiling, it changes from large to small, and when it boils, it changes from small to large.
4. According to the boiling point table of liquids on page P60 of the textbook, what is the boiling point of water? Why is the boiling point of water different from what is given in the textbook when we did the experiment?
5. After the water boils and the alcohol lamp is removed, why does the water continue to boil for a period of time?
communication and evaluation
What are the reasons why experiments succeed or fail?
1. It may be that the temperature of the experimental water is too low
2. It may be that the outer flame of the alcohol lamp was not used to heat the water in the beaker during the experiment.
3. It may be that there is too much heated water in the beaker
Think about it, discuss it
If you apply some alcohol to the glass bulb of a thermometer, what will happen to the thermometer's reading?
First it falls, then it rises, and finally it remains unchanged.
When the alcohol evaporates, it absorbs heat, causing the thermometer's reading to decrease. When the alcohol evaporates, the thermometer's reading increases until it stabilizes.
2. What are the factors that affect the speed of evaporation?
(1) The temperature of the liquid. The higher the temperature, the faster it evaporates.
(2) Surface area of the liquid. The larger the surface area of the liquid, the faster it evaporates.
(3) The air flows on the liquid surface. The faster the air flows on the liquid surface, the faster it evaporates.
1. (2010 Linyi, Shandong) Please determine which of the following images belongs to the boiling image of water ( )
2. (2009 Henan) As shown in the figure, boil water in an open kettle. After the water boils and then continue to heat it with a strong fire, the temperature of the water will be ( )
A. Vaporization accelerates but the temperature decreases
B. It fluctuates with the size of the fire.
C. Gradually increase
D. unchanged
3. (2011 Heze) When drinking boiling water, people often blow air onto the water surface so that it does not taste too hot. This is because ( )
A. Blowing air onto the water surface accelerates the evaporation of water, and water evaporation absorbs heat, lowering the temperature of the water.
B. Blow cold air into the water to lower the water temperature
C. Blow gas to make the hot water sink and the cold water stay at the top.
D. None of the above statements are correct
Keywords: Changes in the state of matter teaching courseware, Vaporization and liquefaction teaching courseware, People's Education Edition eighth grade physics PPT courseware download, eighth grade physics slide courseware download, Changes in the state of matter PPT courseware download, Vaporization and liquefaction PPT courseware download, .PPT Format;
For more information about the PPT courseware "Changes in the State of Matter, Vaporization and Liquefaction", please click on the Changes in the State of Matter ppt, Vaporization and Liquefaction ppt tab.
"Exploring the Characteristics of Vaporization and Liquefaction" Material Forms and Their Changes PPT Courseware 2:
"Exploring the Characteristics of Vaporization and Liquefaction" Material Forms and Changes PPT Courseware 2 Learning Objectives 1. Be able to distinguish between solid, liquid and gaseous states of matter. Know what vaporization and liquefaction are. Know that vaporization absorbs heat and liquefaction releases heat. 2. Know what evaporation is and how it affects evaporation speed..
"Exploring the Characteristics of Vaporization and Liquefaction" Material Forms and Their Changes PPT Courseware:
"Exploring the Characteristics of Vaporization and Liquefaction" Material Forms and Changes PPT Courseware Teaching Objectives 1. Describe the basic characteristics of the three physical states of solid, liquid and gas. List the different states of matter in nature and life and their applications. 2. Know what vaporization and liquefaction are, know vapor...
"Vaporization and Liquefaction" PPT Courseware on Changes in State of Matter 10:
"Vaporization and Liquefaction" PPT Courseware on Changes in State of Matter 10 Think about it. Put a few drops of alcohol into a transparent plastic bag; deflate the bag, drain out the air and tie the mouth tightly with a rope; then put it into a hot pot above 80℃ in water. What changes will you see? Remove the plastic bag from the hot water..
File Info
Update Time: 2024-11-22
This template belongs to Physics courseware People's Education Edition Physics for Grade 8, Volume 1 industry PPT template
"Vaporization and Liquefaction" PPT Courseware on Changes in State of Matter 6 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Vaporization and Liquefaction" PPT Courseware on Changes in State of Matter 6 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Vaporization and Liquefaction" PPT Courseware on Changes in State of Matter 6, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview