Fifth Grade Science Volume 1, Textbook Edition
Science Edition for Sixth Grade Science Volume 2
Science Edition for Sixth Grade Science Volume 1
Third Grade Science Volume 2, Textbook Edition
Third Grade Science Volume 1, Textbook Edition
Fourth Grade Science Volume 2, Textbook Edition
Fourth Grade Science Volume 1, Textbook Edition
Fourth-grade science volume 2 of the E-education edition
Qingdao Edition Fourth Grade Science Volume 2
Hunan Education Edition Fourth Grade Science Volume 1
E-education edition fifth grade science volume 1
E-education edition fifth grade science volume 2
E-education edition sixth grade science volume 1
Fifth Grade Science Volume 2, Textbook Edition
Qingdao Edition Fourth Grade Science Volume 1
People's Education Press Fourth Grade Science Volume 2

| Category | Format | Size |
|---|---|---|
| Zhejiang Education Edition Ninth Grade Science Volume 1 | pptx | 6 MB |
Description
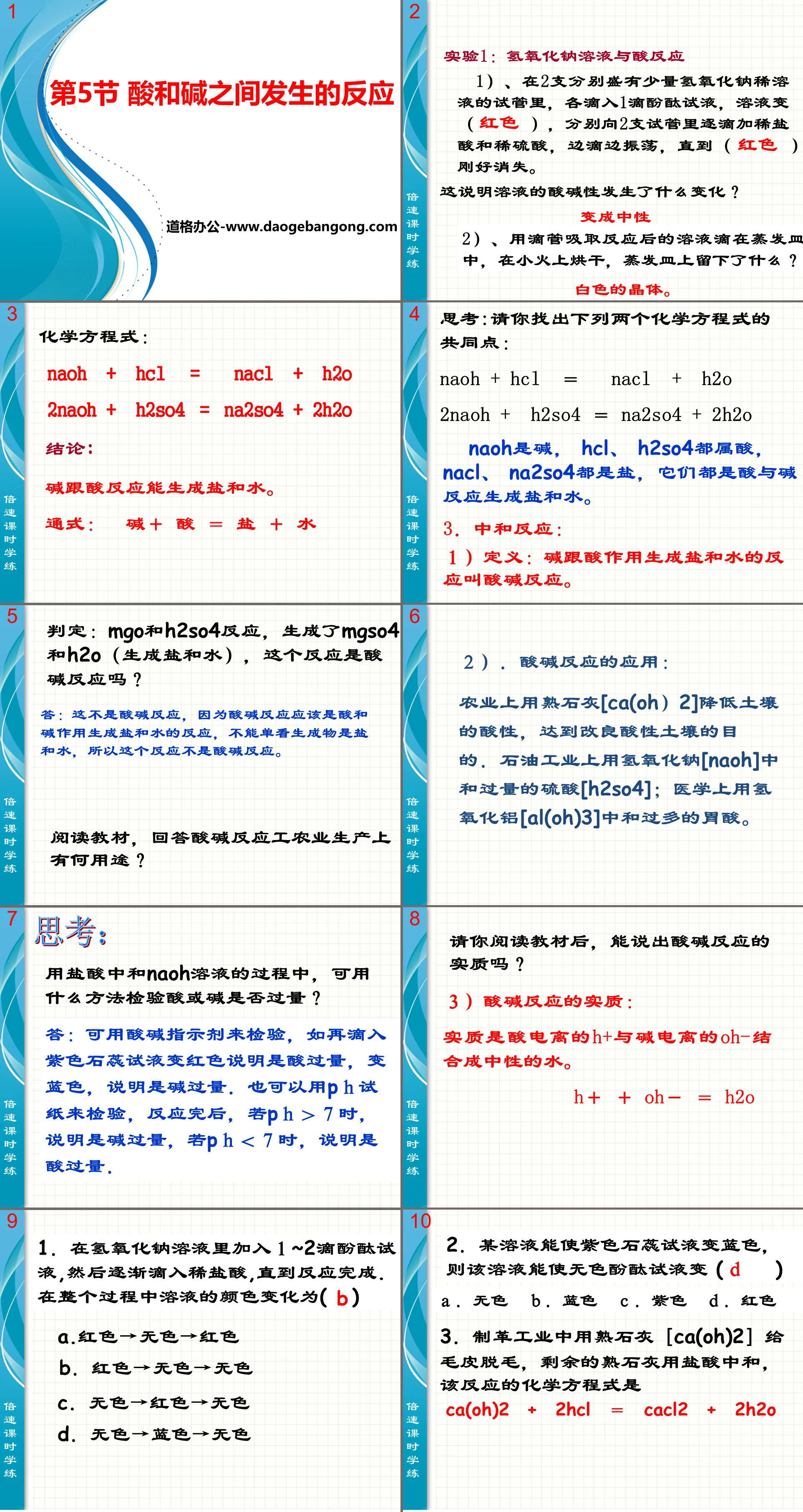
"Reaction between Acids and Bases" PPT courseware
Part One Content: Classroom Experiment
Experiment 1: Reaction of sodium hydroxide solution with acid
1). In two test tubes containing a small amount of dilute sodium hydroxide solution, drop 1 drop of phenolphthalein test solution in each. The solution becomes ( ). Add dilute hydrochloric acid and dilute sulfuric acid dropwise to the two test tubes respectively while dripping. Oscillate until ( ) just disappears.
What does this mean about the change in the acidity and alkalinity of the solution?
become neutral
2) Use a dropper to absorb the reacted solution and drop it into an evaporating dish. Dry it on a low fire. What is left on the evaporating dish?
White crystals.
Chemical equation:
NaOH + HCl = NaCl + H2O
2NaOH + H2SO4 = Na2SO4 + 2H2O
in conclusion:
Bases react with acids to form salt and water.
General formula: base + acid = salt + water
Reaction between acid and base PPT, Part 2: Neutralization reaction:
1) Definition: The reaction in which a base reacts with an acid to form salt and water is called an acid-base reaction.
Judgment: MgO and H2SO4 react to produce MgSO4 and H2O (salt and water are produced). Is this reaction an acid-base reaction?
Answer: This is not an acid-base reaction, because an acid-base reaction should be a reaction in which an acid and a base react to form salt and water. We cannot just think that the products are salt and water, so this reaction is not an acid-base reaction.
2) Application of acid-base reaction:
In agriculture, hydrated lime "Ca(OH)2" is used to reduce the acidity of soil and achieve the purpose of improving acidic soil. In the petroleum industry, sodium hydroxide [NaOH] is used to neutralize excess sulfuric acid [H2SO4]; in medicine, aluminum hydroxide [Al(OH)3] is used to neutralize excess sulfuric acid. Too much stomach acid.
3) The essence of acid-base reaction:
The essence is that acid ionized H+ and alkali ionized OH- combine to form neutral water.
H++OH-=H2O
Reaction between acids and bases PPT, Part 3: Classroom exercises
1. Add 1 to 2 drops of phenolphthalein test solution to the sodium hydroxide solution, and then gradually drop in dilute hydrochloric acid until the reaction is completed. The color change of the solution during the entire process is ()
A. red → colorless → red
B. Red→Colorless→Colorless
C. Colorless → red → colorless
D. Colorless → blue → colorless
2. A certain solution can turn purple litmus test solution blue, then the solution can turn colorless phenolphthalein test solution ( )
A. Colorless b. Blue C. Purple D. red
3. In the tanning industry, hydrated lime [Ca(OH)2] is used to depilate fur, and the remaining hydrated lime is neutralized with hydrochloric acid. The chemical equation of this reaction is
Ca(OH)2+2HCl=CaCl2+2H2O
Keywords: Zhejiang Education Edition ninth grade science PPT courseware free download, reaction between acids and bases PPT download, .PPT format;
For more information about the PPT courseware "Reactions between Acids and Bases", please click on the Reactions between Acids and Bases ppt tab.
"Reaction between Acids and Bases" PPT:
"Reaction between Acids and Bases" PPT Part One Content: Learning Objectives: 1. Find evidence of the reaction between acids and bases through independent exploration; 2. Give examples of the applications of acid-base reactions in life. Scenarios and questions raised Acid is acidic, with pH <7, and can make purple...
File Info
Update Time: 2024-10-12
This template belongs to science courseware Zhejiang Education Edition Ninth Grade Science Volume 1 industry PPT template
"Reaction between Acids and Bases" PPT courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Reaction between Acids and Bases" PPT courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Reaction between Acids and Bases" PPT courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview