| Category | Format | Size |
|---|---|---|
| Zhejiang Education Edition Ninth Grade Science Volume 1 | pptx | 6 MB |
Description
"Chemical Properties of Metals" PPT (Lesson 3)
Part One: Teaching Objectives
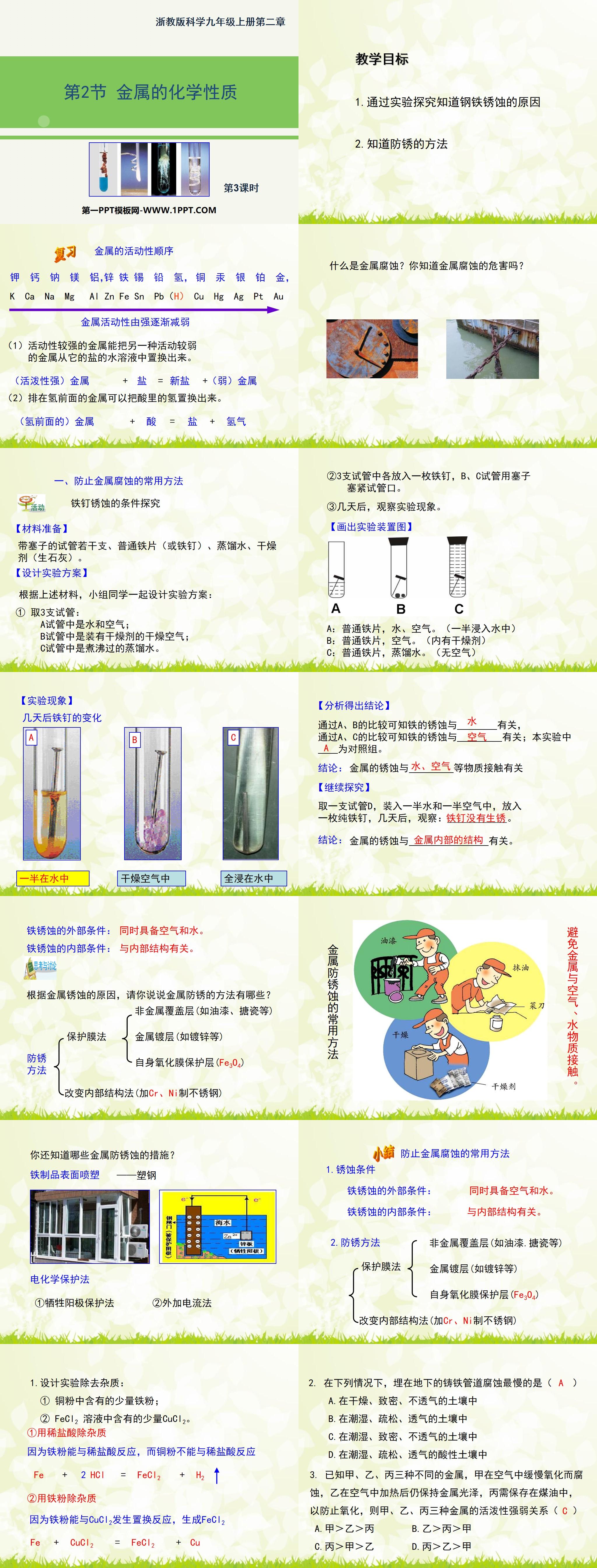
1. Understand the causes of steel corrosion through experiments
2. Know how to prevent rust
Chemical Properties of Metals PPT, Part 2: Common Methods to Prevent Metal Corrosion
Research on the conditions of iron nail rust
【Material preparation】
Several test tubes with stoppers, ordinary iron sheets (or iron nails), distilled water, and desiccant (quick lime).
[Design experimental plan]
Based on the above materials, students in groups design an experimental plan together:
① Take 3 test tubes:
A test tube contains water and air;
Test tube B contains dry air containing desiccant;
Test tube C contains boiled distilled water.
②Put an iron nail into each of the three test tubes, and plug the mouths of test tubes B and C with stoppers.
③After a few days, observe the experimental phenomena.
A: Ordinary iron sheets, water and air. (halfway immersed in water)
B: Ordinary iron sheet, air. (Contains desiccant)
C: Ordinary iron sheets, distilled water. (no air)
Chemical Properties of Metals PPT, Part 3: Classroom Exercises
1. Design experiments to remove impurities:
① A small amount of iron powder contained in copper powder;
② FeCl2 contains a small amount of CuCl2 in the solution.
①Use dilute hydrochloric acid to remove impurities
Because iron powder can react with dilute hydrochloric acid, but copper powder cannot react with dilute hydrochloric acid
② Use iron powder to remove impurities
Because iron powder can undergo a displacement reaction with CuCl2 to generate FeCl2
2. Under the following circumstances, the cast iron pipes buried underground will corrode the slowest: ( )
A. In dry, dense, air-impermeable soil
B. In moist, loose and breathable soil
C. In moist, dense, air-impermeable soil
D. In moist, loose, breathable acidic soil
3. It is known that A, B, and C are three different metals. A slowly oxidizes and corrodes in the air, B still maintains its metallic luster after being heated in the air, and C needs to be stored in kerosene to prevent oxidation, then A, B The relationship between the reactivity of the three metals, acrylic and acrylic ( )
A.A>B>C B.B>C>A
C.C>A>B D.C>B>A
Keywords: Zhejiang Education Edition ninth grade science PPT courseware free download, chemical properties of metal PPT download, .PPT format;
For more information about the "Chemical Properties of Metals" PPT courseware, please click on the "Chemical Properties of Metals" ppt tab.
"Chemical Properties of Metals" PPT courseware:
"Chemical Properties of Metals" PPT courseware Part One: Chemical Properties of Metals Knowledge Review: What are the chemical properties of metals? Iron will rust, and zinc can react with hydrochloric acid to release hydrogen. These are the chemical properties of metals. ①The reaction between metal and oxygen..
"Chemical Properties of Metals" PPT (second lesson):
"Chemical Properties of Metals" PPT (Second Lesson) Part One Content: Teaching Objectives: 1. Understand the activity sequence of metals through the reactions of typical metals with acids and certain salts; 2. Understand the characteristics of displacement reactions and understand the electrons in role in chemical reactions. ... ....
"Chemical Properties of Metals" PPT (first lesson):
"Chemical Properties of Metals" PPT (First Lesson) Part One Content: Teaching Objectives 1. Know the chemical properties of metals, which can react with oxygen and react with acids; 2. Know the definition of displacement reaction. Think and discuss What are the main chemical properties of metals? ..
File Info
Update Time: 2024-07-30
This template belongs to science courseware Zhejiang Education Edition Ninth Grade Science Volume 1 industry PPT template
"Chemical Properties of Metals" PPT (Lesson 3) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Chemical Properties of Metals" PPT (Lesson 3) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Chemical Properties of Metals" PPT (Lesson 3), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview