Fifth Grade Science Volume 1, Textbook Edition
Science Edition for Sixth Grade Science Volume 2
Science Edition for Sixth Grade Science Volume 1
Third Grade Science Volume 2, Textbook Edition
Fourth Grade Science Volume 2, Textbook Edition
Fourth Grade Science Volume 1, Textbook Edition
Third Grade Science Volume 1, Textbook Edition
Fourth-grade science volume 2 of the E-education edition
Qingdao Edition Fourth Grade Science Volume 2
Hunan Education Edition Fourth Grade Science Volume 1
E-education edition fifth grade science volume 1
E-education edition fifth grade science volume 2
Fifth Grade Science Volume 2, Textbook Edition
E-education edition sixth grade science volume 1
Zhejiang Education Edition Seventh Grade Science Volume 2
People's Education Press Fifth Grade Science Volume 2
| Category | Format | Size |
|---|---|---|
| Zhejiang Education Edition Eighth Grade Science Volume 2 | pptx | 6 MB |
Description
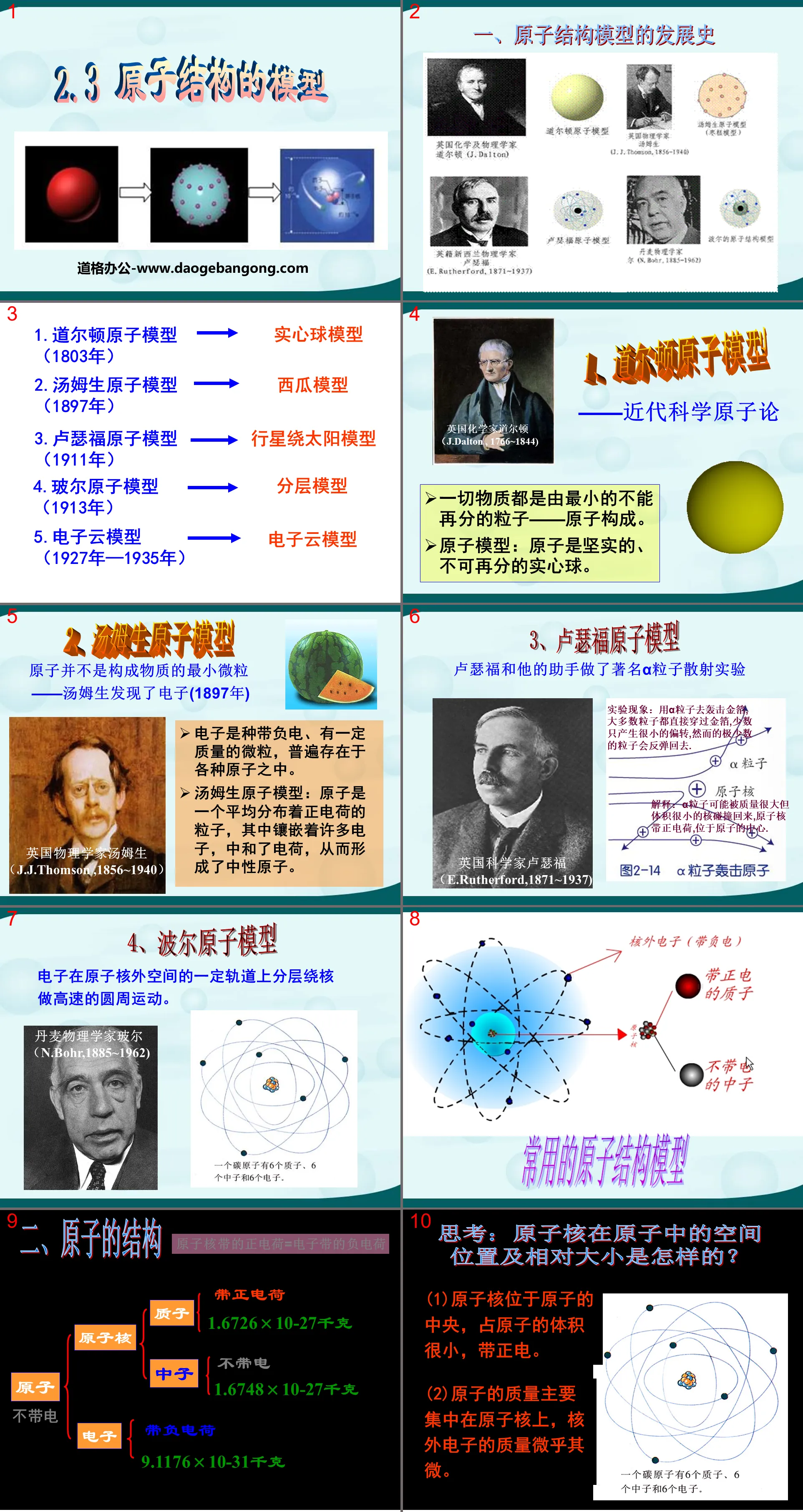
"Model of Atomic Structure" PPT
Part One: The Development History of Atomic Structural Models
1. Dalton Atomic Model
——Modern scientific atomism
All matter is composed of the smallest indivisible particles - atoms.
Atomic model: Atoms are solid, irreducible solid balls.
2. Thomson’s atomic model
Atoms are not the smallest particles that make up matter - Thomson discovered the electron (1897)
Electrons are negatively charged particles with a certain mass that are commonly found in various atoms.
Thomson's atomic model: An atom is a particle with an evenly distributed positive charge in which many electrons are embedded, neutralizing the charge, thus forming a neutral atom.
3. Rutherford’s atomic model
Rutherford and his assistants conducted the famous alpha particle scattering experiment
Experimental phenomenon: Use alpha particles to bombard gold foil. Most of the particles will pass through the gold foil directly, a few will only produce a small deflection, but a very small number of particles will bounce back.
Explanation: The alpha particle may be collided back by a nucleus with a large mass but a small volume. The nucleus is positively charged and is located at the center of the atom.
4. Bohr atomic model
The electrons perform high-speed circular motion around the nucleus in layers in a certain orbit in the space outside the nucleus.
Model PPT of atomic structure, part 2: structure of atom
Positive charge on the nucleus = Negative charge on the electrons
Thinking: What is the spatial position and relative size of the nucleus in the atom?
(1) The nucleus is located in the center of the atom, occupies a small volume of the atom, and is positively charged.
(2) The mass of an atom is mainly concentrated in the nucleus, and the mass of electrons outside the nucleus is very small.
(3), in the same atom:
Nuclear charge = Number of protons = Number of electrons outside the nucleus ≠ Number of neutrons
Atomic structure model PPT, the third part: summary:
1. Atoms are composed of a nucleus at the center of the atom and electrons outside the nucleus;
2. The nucleus of an atom is positively charged, and the electrons outside the nucleus are negatively charged;
3. The nucleus is very small, but almost contains the mass of the entire atom;
4. The electrons outside the nucleus have a very small mass and are layered around the nucleus and moving at high speed.
Keywords: Zhejiang Education Edition eighth grade science PPT courseware for the second volume free download, atomic structure model PPT download, .PPT format;
For more information about the "Model of Atomic Structure" PPT courseware, please click the "Model of Atomic Structure ppt" tab.
File Info
Update Time: 2024-11-21
This template belongs to science courseware Zhejiang Education Edition Eighth Grade Science Volume 2 industry PPT template
"Model of Atomic Structure" PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Model of Atomic Structure" PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Model of Atomic Structure" PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview