People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
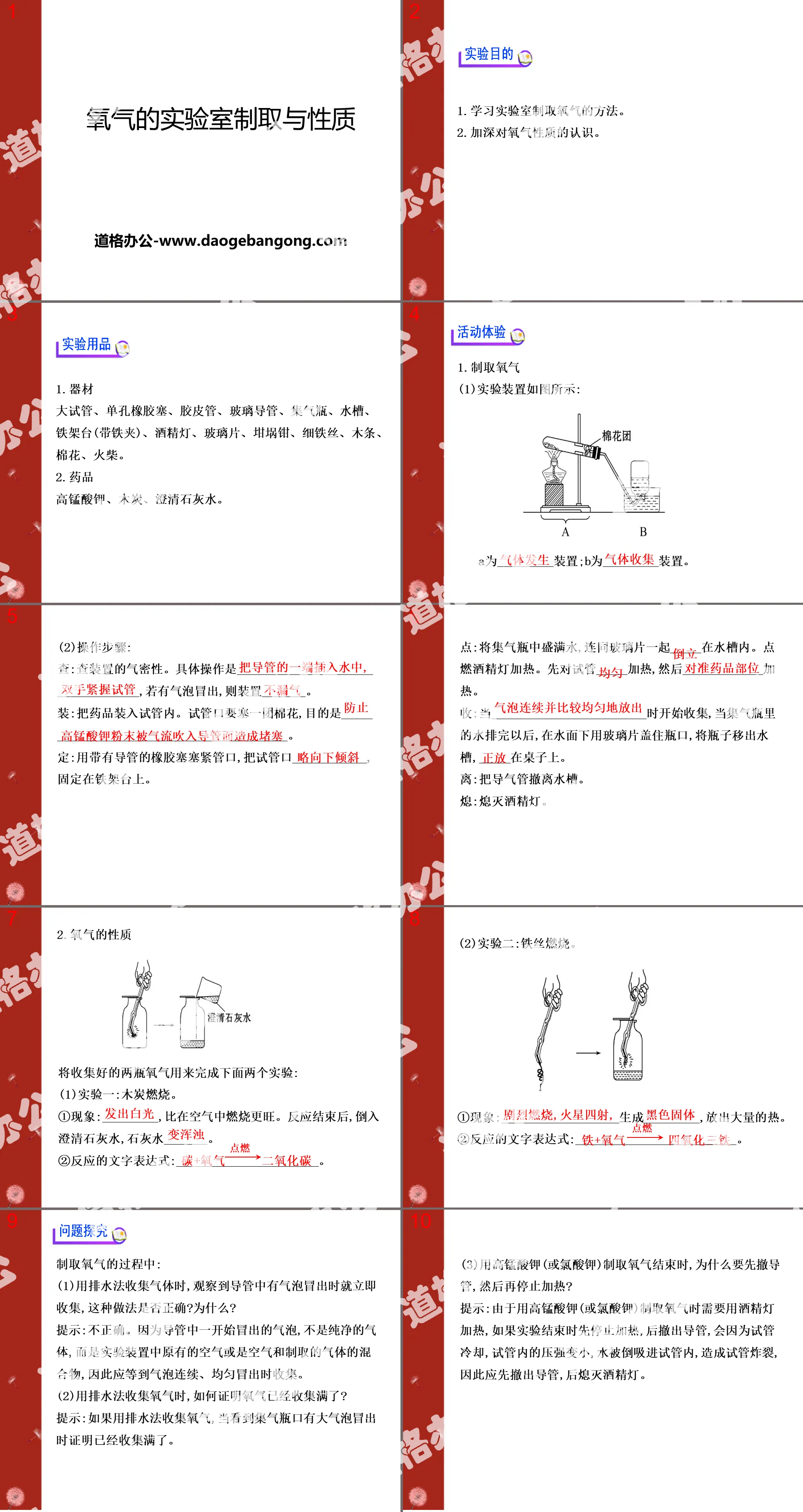
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 4
Purpose
1. Learn how to produce oxygen in the laboratory.
2. Deepen your understanding of the properties of oxygen.
Experimental supplies
1. Equipment
Large test tube, single-hole rubber stopper, rubber tube, glass tube, gas bottle, sink, iron stand (with iron clamp), alcohol lamp, glass piece, crucible tongs, thin wire, wooden strips, cotton, matches.
2. Medicines
Potassium permanganate, charcoal, clarified lime water.
activity experiment
1. Preparation of oxygen
(1) The experimental device is as shown in the figure:
A is a ________ device; B is a ________ device.
(2) Operation steps:
Check: Check the air tightness of the device. The specific operation is ___________. If bubbles appear, install _______.
Loading: Put the medicine into the test tube. A ball of cotton should be stuffed into the mouth of the test tube for the purpose of _______________________________.
Fix: Plug the mouth of the test tube tightly with a rubber stopper with a conduit, and fix the mouth of the test tube on the iron stand.
2. Properties of oxygen
Use the collected two bottles of oxygen to complete the following two experiments:
(1) Experiment 1: Charcoal burning.
① Phenomenon: ________, burning more vigorously than in air. After the reaction is completed, pour
Clarified lime water, lime water _______.
②The text expression of the reaction: ____________________.
(2) Experiment 2: Iron wire burning.
① Phenomenon: ____________________ generates _________ and releases a large amount of heat.
②The text expression of the reaction: ____________________.
In the process of producing oxygen:
(1) When using the drainage method to collect gas, collect it immediately when you observe bubbles emerging from the tube. Is this correct? Why?
Tip: Incorrect. Because the bubbles that initially emerge from the tube are not pure gas, but the original air in the experimental device or a mixture of air and produced gas, you should wait until the bubbles emerge continuously and evenly to collect them.
(2) When using the drainage method to collect oxygen, how to prove that the oxygen has been fully collected?
Tip: If you use the drainage method to collect oxygen, when you see big bubbles coming out of the mouth of the gas collecting bottle, it proves that the collection is full.
(3) When using potassium permanganate (or potassium chlorate) to produce oxygen, why do you need to withdraw the catheter first and then stop heating?
Tip: Since potassium permanganate (or potassium chlorate) is used to produce oxygen, it needs to be heated with an alcohol lamp. If the heating is stopped at the end of the experiment and then the tube is withdrawn, the pressure in the test tube will become smaller due to the cooling of the test tube, and the water will be poured out. It may be sucked into the test tube and cause the test tube to burst. Therefore, the tube should be withdrawn first and then the alcohol lamp should be extinguished.
Experimental drill
1. Potassium permanganate is used to produce oxygen in the laboratory. After collection, the correct sequence for disassembling the device is ()
①Remove the alcohol lamp ②Take out the catheter from the sink
③Remove the test tube from the iron stand
A.①③②B.③②①
C.②①③ D.①②③
[Analysis] Choose C. After the collection is completed, the correct sequence for disassembling the device is: take out the catheter from the sink, remove the alcohol lamp, and remove the test tube from the iron stand.
2. A classmate used potassium permanganate as raw material to produce oxygen and collected it using the drainage method. However, the oxygen was not collected after heating for a long period of time. The possible reason is ()
A. Too little potassium permanganate B. No cotton added to the test tube mouth
C. The air tightness of the device is not good D. The heating temperature is not high
[Analysis] Choose C. If no oxygen is collected after heating for a long period of time, it means that the device is leaking, that is, the air tightness of the device is not good.
3. In the device for producing oxygen with potassium permanganate in the laboratory, which statement about several "mouths" is wrong ()
A. The mouth of the test tube containing drugs should be upward
B. The opening of the air guide tube entering the test tube should expose a little of the single-hole plug.
C. When using the exhaust method to collect oxygen, the mouth of the gas collecting bottle should be facing upward.
D. When using the upward air exhaust method to collect oxygen, the mouth of the air guide should be extended into the bottom of the gas collecting bottle
[Analysis] Choose A. The mouth of the test tube should be tilted slightly downward to prevent condensed water from flowing back into the test tube and causing the test tube to burst.
4. The picture below is a diagram of a laboratory device for heating potassium permanganate to produce oxygen. Which of the following analysis of experimental operations is incorrect ()
A. Air tightness check: Hold the test tube tightly with your hand and observe bubbles emerging from the air tube in the water, indicating that the device does not leak.
B. The mouth of the test tube is slightly tilted downward: to prevent water on the wall of the test tube from flowing into the bottom of the test tube and causing the test tube to burst.
C. Heating: Directly use the flame of an alcohol lamp to heat the location of the medicine.
D. Stop heating: first move the catheter out of the water, then extinguish the alcohol lamp
[Analysis] Choose C. When using potassium permanganate to produce oxygen, tilt the test tube mouth slightly downward; preheat it first when heating. If you directly use the flame of an alcohol lamp to heat the location of the medicine, the test tube will be heated unevenly and explode; at the end of the experiment, first Remove the catheter and then remove the alcohol lamp.
Keywords: Laboratory Preparation and Properties of Oxygen Teaching Courseware, Hunan Education Edition Ninth Grade Chemistry Volume 1 Chemistry PPT Courseware Download, Ninth Grade Chemistry Slide Courseware Download, Oxygen Laboratory Preparation and Properties PPT Courseware Download, .PPT format;
For more information about the "Laboratory Preparation and Properties of Oxygen" PPT courseware, please click on the "Laboratory Preparation and Properties of Oxygen" ppt tab.
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 3:
"Laboratory Preparation and Properties of Oxygen" PPT courseware 3 1. Producing oxygen by heating potassium permanganate 1. Experiment purpose: Preparing oxygen and exploring the properties of oxygen 2. Experimental principle: 3. Instruments and drugs: iron stand, Iron clamps, test tubes, single-hole rubber stoppers, air tubes, collection...
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 2:
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 2 Learning Objectives 1. Practice the method of producing and collecting oxygen in the laboratory. 2. Verify the chemical properties of oxygen by burning charcoal and iron wire. Pre-study tutorial 1. Prepare experimental instruments and drugs, iron stand (with iron clamps), test tubes...
"Laboratory Preparation and Properties of Oxygen" PPT courseware:
"Laboratory Preparation and Properties of Oxygen" PPT courseware Experiment purpose: 1. Master the principles and experimental equipment of laboratory preparation of oxygen. 2. Preliminarily learn how to use hydrogen peroxide to produce three bottles of oxygen, and verify the properties of oxygen. Think: We make oxygen in the laboratory...
File Info
Update Time: 2024-11-22
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 4 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Laboratory Preparation and Properties of Oxygen" PPT Courseware 4 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 4, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview