People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Lu Ke Edition High School Chemistry Compulsory Course 1 | pptx | 6 MB |
Description
"Classification of Elements and Matter" Elements and the Material World PPT (Lesson 1: Relationship between Elements and Matter, Classification of Matter)
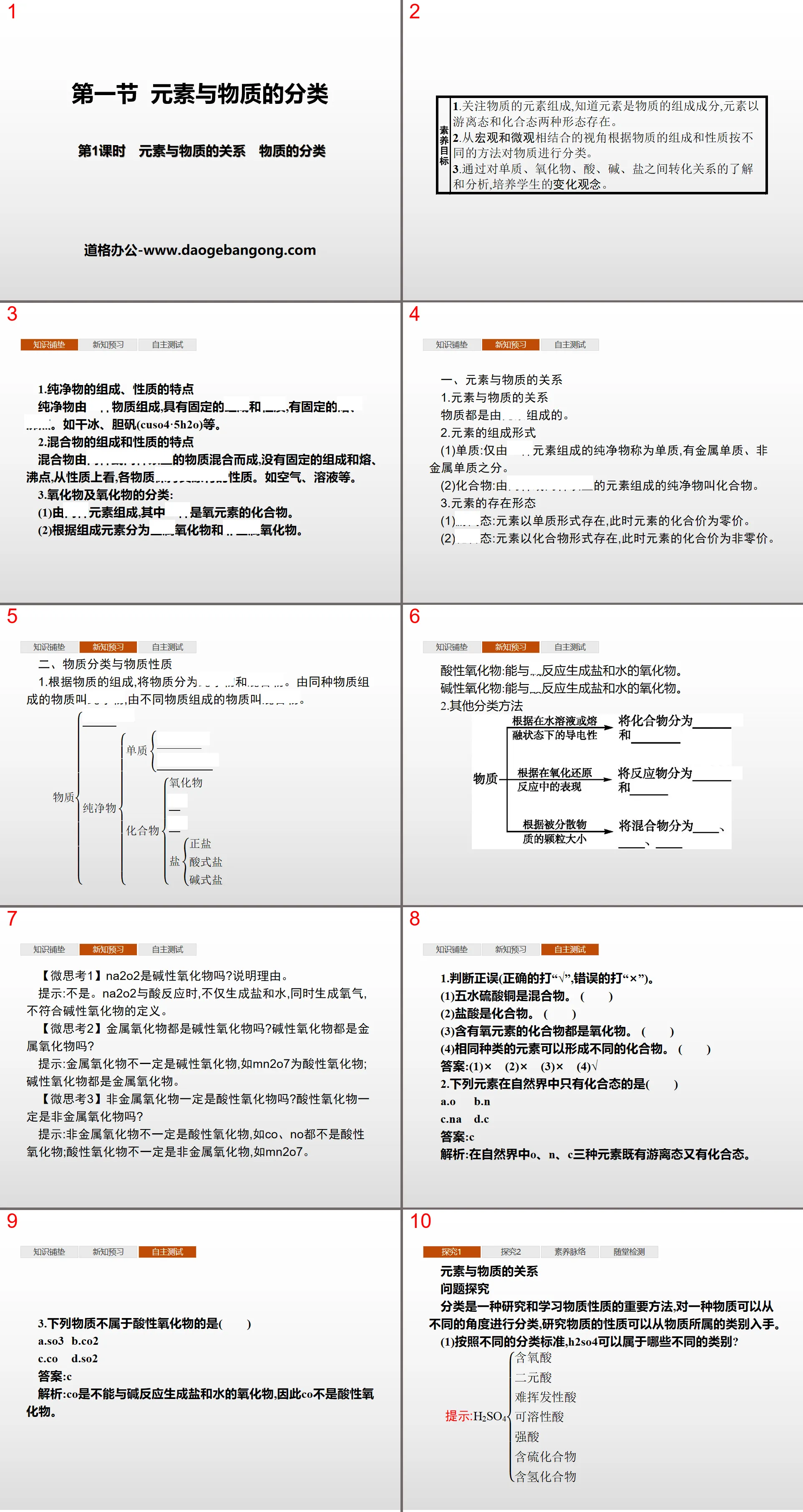
Part One: Literacy Goals
1. Pay attention to the elemental composition of matter and know that elements are the components of matter. Elements exist in two forms: free state and combined state.
2. Classify substances in different ways according to their composition and properties from a combined macroscopic and microscopic perspective.
3. Cultivate students' concepts of change through understanding and analysis of the transformation relationships between elements, oxides, acids, bases, and salts.
Classification of elements and substances PPT, part 2 content: knowledge foundation
1. The composition and properties of pure substances
Pure substances are composed of a substance with fixed composition and properties, and fixed melting and boiling points. Such as dry ice, bile alum (CuSO4·5H2O), etc.
2. Characteristics of the composition and properties of the mixture
A mixture is composed of two or more substances and has no fixed composition, melting and boiling points. In terms of properties, each substance maintains its original properties. Such as air, solution, etc.
3. Classification of oxides and oxides:
(1) It is composed of two elements, one of which is a compound of oxygen element.
(2) According to the constituent elements, it is divided into metal oxides and non-metal oxides.
Classification of elements and substances PPT, part three: preview of new knowledge
1. The relationship between elements and matter
1. The relationship between elements and matter
Matter is composed of elements.
2. The composition of elements
(1) Elemental substance: A pure substance composed of only one element is called an elemental substance, which can be divided into metallic elemental elements and non-metallic elemental elements.
(2) Compound: A pure substance composed of two or more elements is called a compound.
3. The existence form of elements
(1) Free state: The element exists in elemental form, and the valence of the element at this time is zero.
(2) Combined state: The element exists in the form of a compound, and the valence of the element is non-zero at this time.
2. Material classification and material properties
1. According to the composition of substances, substances are divided into pure substances and mixtures. Substances composed of the same substance are called pure substances, and substances composed of different substances are called mixtures.
Acidic oxides: Oxides that react with bases to form salts and water.
Alkaline oxide: An oxide that reacts with acid to form salt and water.
2. Other classification methods
[Micro Thought 1] Is Na2O2 an alkaline oxide? Explain the reason.
Hint: No. When Na2O2 reacts with acid, it not only generates salt and water, but also generates oxygen, which does not meet the definition of an alkaline oxide.
[Micro Thought 2] Are all metal oxides alkaline oxides? Are all alkaline oxides metal oxides?
Tip: Metal oxides are not necessarily alkaline oxides. For example, Mn2O7 is an acidic oxide; alkaline oxides are all metal oxides.
[Micro Thought 3] Are non-metal oxides necessarily acidic oxides? Are acidic oxides necessarily non-metal oxides?
Tip: Non-metal oxides are not necessarily acidic oxides, such as CO and NO, which are not acidic oxides; acidic oxides are not necessarily non-metal oxides, such as Mn2O7.
Classification of elements and substances PPT, part 4: independent testing
1. Judge whether it is right or wrong (mark “√” if it is correct and “×” if it is wrong).
(1) Copper sulfate pentahydrate is a mixture. ()
(2) Hydrochloric acid is a compound. ()
(3) Compounds containing oxygen elements are all oxides. ()
(4) The same kind of elements can form different compounds. ()
Answer: (1)× (2)× (3)× (4)√
2. The following elements are found only in the chemical state in nature ()
A.O.B.N.
C.Na D.C
Answer:C
Analysis: In nature, the three elements O, N, and C exist in both free and combined states.
3. Which of the following substances is not an acidic oxide ()
A.SO3 B.CO2
C.CO D.SO2
Answer:C
Analysis: CO is an oxide that cannot react with alkali to form salt and water, so CO is not an acidic oxide.
Classification of elements and substances PPT, part 5: exploration
The relationship between elements and matter
Question exploration
Classification is an important method for studying and learning the properties of matter. A substance can be classified from different perspectives. To study the properties of a substance, we can start from the category to which the substance belongs.
(1) According to different classification standards, what different categories can H2SO4 belong to?
(2) List the similarities and differences in the properties of hydrochloric acid and sulfuric acid.
Tip: The properties of hydrochloric acid and sulfuric acid are similar: they both have acid properties.
Difference: Sulfuric acid contains sulfate ions, which can form BaSO4 white precipitate with Ba2+. Hydrochloric acid contains chloride ions, which can form a white precipitate of AgCl with Ag+. (As long as the answer is reasonable)
Knowledge induction
1. The relationship between elements, substances and particles
(1) Composition of matter
On a macroscopic level, matter is composed of elements, and on a microscopic level, matter is composed of molecules, atoms, and ions.
A general term for a type of atoms whose elements have the same nuclear charge. Elements exist in nature in free state and combined state
Molecules are the smallest particles that maintain the chemical properties of matter
Atoms are the smallest particles involved in chemical changes. Atoms are composed of a nucleus and electrons outside the nucleus, in which the nucleus is composed of protons and neutrons (except for individual atoms)
An ion is an electrically charged atom or group of atoms. Ions are divided into cations and anions
(2) Relationship diagram between elements, substances and particles
2. Classification of substances
Classification methods are generally divided into cross classification (classifying substances according to different standards) and tree classification (reclassifying similar things).
(1) Cross classification method - classify substances from different angles. like:
(2) Tree classification method - classify substances step by step according to different levels, such as:
Misunderstandings: Be wary of the "four major misunderstandings" in the composition and classification of substances.
(1) Mistakenly believing that substances composed of the same elements must be pure substances. A substance may be a mixture of different elements of an element. For example, a mixed gas composed of O2 and O3 is a mixture.
(2) Mistakenly believe that the substance that can dissociate H+ must be an acid, such as NaHSO4 is a salt.
(3) Mistakenly believe that acidic oxides must be non-metal oxides. For example, Mn2O7 is a metal oxide but is an acidic oxide. Non-metal oxides are not necessarily acidic oxides. For example, CO and NO are not acidic oxides.
(4) Mistakenly believe that metal oxides must be alkaline oxides. For example, Na2O2 is a peroxide, not an alkaline oxide.
Classification of elements and substances PPT, Part 6: In-class testing
1. Which of the following substances are pure substances, compounds, or calcium salts ()
A. Quick lime B. Hydrated lime C. Calcium carbonate D. Limestone
Answer:C
Analysis: Quicklime and hydrated lime are not salts. The main component of limestone is CaCO3, which is not a pure substance.
2. The substance that matches the shaded part in the picture is ()
A.KNO3 B.KHSO4
C.K2S D.K2SO4
Answer:D
Analysis: KNO3 belongs to normal salts and potassium salts, but not sulfates, so A is wrong; KHSO4 belongs to acid salts, potassium salts, and sulfates, but does not belong to normal salts, so B is wrong; K2S belongs to normal salts, potassium salts, But it does not belong to sulfate, so C is wrong; K2SO4 belongs to normal salt, potassium salt, and sulfate, so D is correct.
3. Which of the following substances is incorrectly classified ()
A. Water, hydrogen peroxide and dry ice are all oxides
B.H2SO4, HNO3, and H2CO3 are all acids
C. Caustic soda, soda ash, and hydrated lime are alkalis
D.NaHSO4, CuSO4 and KMnO4 are all salts
Answer:C
Analysis: An oxide is a compound composed of two elements, one of which is oxygen. Water (H2O), hydrogen peroxide (H2O2) and dry ice (CO2) are all oxides, so A is correct; they dissociate in aqueous solution Compounds whose cations are all hydrogen ions are acids. H2SO4, HNO3, and H2CO3 are all acids, so B is correct; caustic soda (NaOH) is an alkali, soda ash (Na2CO3) is a salt, and slaked lime is Ca(OH)2. 093; Belongs to a base, so C is wrong; Compounds that can dissociate metal cations (or ammonium ions) and acid ions are salts. NaHSO4, CuSO4 and KMnO4 are all salts, so D is correct.
4. Which of the following judgments about oxides is correct ()
A. All compounds containing oxygen elements can be called oxides
B. According to different properties, oxides can only be divided into acidic oxides and basic oxides.
C. Acidic oxides can react with bases to form salts and water
D. Metal oxides must be alkaline oxides
Answer:C
Analysis: Oxides are compounds containing only two elements, one of which is oxygen, such as CO2, Na2O, etc. Compounds containing oxygen elements are not necessarily oxides, such as H2SO4, KMnO4, etc., so item A is wrong; it can Oxides that react with alkali to form salt and water are called acidic oxides, and oxides that react with acids to form salt and water are called alkaline oxides. NO, CO, etc. are neither acidic oxides nor alkaline oxides. , so item B is wrong and item C is correct; metal oxides are not necessarily alkaline oxides, such as Mn2O7 is an acidic oxide, so item D is wrong.
Keywords: Free download of PPT courseware for Lu Ke version of high school chemistry compulsory course one, PPT download of the classification of elements and substances, PPT download of elements and the world of matter, PPT download of the relationship between elements and substances, PPT download of classification of substances, .PPT format;
For more information about the PPT courseware "Elements and the Material World, Classification of Elements and Matter, Classification of Matter, Relationship between Elements and Matter", please click on the Elements and Material World ppt Classification of Elements and Matter ppt Classification of Matter ppt Relationship between Elements and Matter ppt tag.
"Integration of this Chapter" Elements and the Material World PPT:
"Integration of this Chapter" Elements and the World of Matter PPT Part 1 Content: Breakthrough Judgment of Large Coexistence of One Ion Example 1 A group of ions that can coexist in large quantities in both strongly acidic and strongly alkaline solutions is ( ) A.Na+, Cu2+, Cl -, SO_4^2- B.K+, Ca2+, NO_3^- ..
"Integration and Improvement at the End of Chapter" Elements and Material World PPT:
"End-of-Chapter Integration Improvement" Elements and Material World PPT Part One Contents: 1. Classification methods and their application in life 1. Classification of elements and substances (1) Elements exist in nature in free and combined states, and very active elements can only exist in combined states. like..
"Micro Project Scientific Use of Chlorine-Containing Disinfectants" Elements and the World of Matter PPT courseware:
"Micro Project Scientific Use of Chlorine-containing Disinfectants" Elements and the World of Matter PPT courseware Part One: Literacy Objectives 1. Predict the properties of chlorine-containing disinfectants from the perspective of substance categories and element valences. 2. Understand the common chlorine-containing disinfectants in daily life and use redox...
File Info
Update Time: 2024-11-21
This template belongs to Chemistry courseware Lu Ke Edition High School Chemistry Compulsory Course 1 industry PPT template
"Classification of Elements and Matter" Elements and the Material World PPT (Lesson 1: Relationship between Elements and Matter, Classification of Matter) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Classification of Elements and Matter" Elements and the Material World PPT (Lesson 1: Relationship between Elements and Matter, Classification of Matter) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Classification of Elements and Matter" Elements and the Material World PPT (Lesson 1: Relationship between Elements and Matter, Classification of Matter), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview