People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Lu Ke Edition High School Chemistry Compulsory Course 1 | pptx | 6 MB |
Description
"Redox Reactions" Elements and the World of Matter PPT Courseware (Understanding Redox Reactions in Lesson 1)
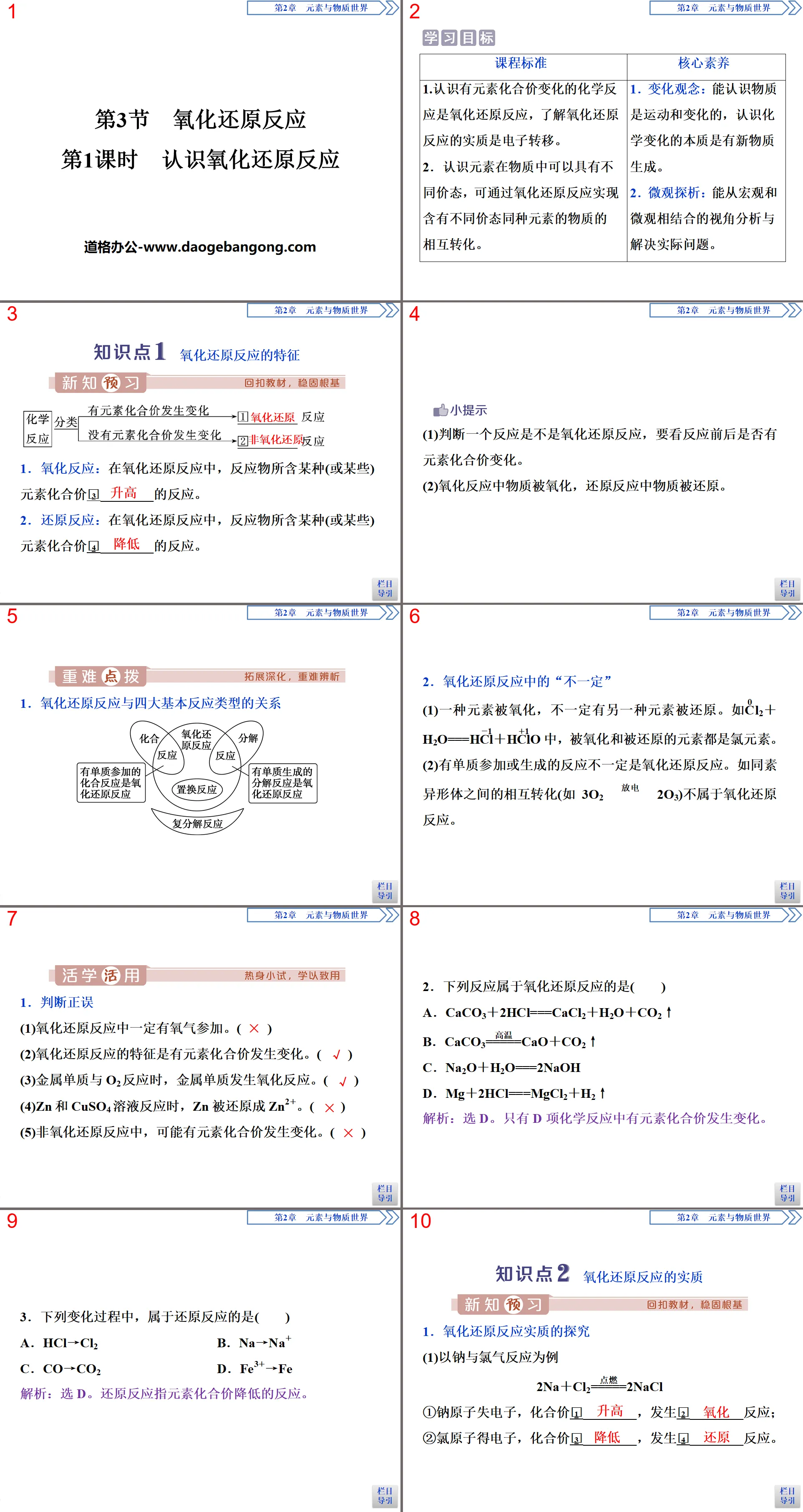
Part One: Learning Objectives
Curriculum Standards
1. Understand that chemical reactions involving changes in the valence of elements are redox reactions, and understand that the essence of redox reactions is electron transfer.
2. Understand that elements can have different valence states in substances, and substances containing the same element in different valence states can be transformed into each other through oxidation-reduction reactions.
core competencies
1. Concept of change: Be able to understand that matter moves and changes, and understand that the essence of chemical change is the generation of new matter.
2. Micro analysis: Able to analyze and solve practical problems from a combination of macro and micro perspectives.
Redox Reaction PPT, Part 2: Knowledge Point 1 Characteristics of Redox Reaction
1. Oxidation reaction: In a redox reaction, a reaction in which the valence of some (or some) elements contained in the reactant is 3___________.
2. Reduction reaction: In an oxidation-reduction reaction, a reaction in which a certain element (or elements) contained in the reactant has a valence of 4___________.
hint
(1) To determine whether a reaction is a redox reaction, it depends on whether there is a change in the valence of the element before and after the reaction.
(2) In the oxidation reaction, the substance is oxidized, and in the reduction reaction, the substance is reduced.
Troubleshooting
1. The relationship between redox reactions and the four basic reaction types
2. "Not necessarily" in redox reactions
(1) When one element is oxidized, another element will not necessarily be reduced. For example, in C0l2+H2O===HCl-1+HCl+1O, the oxidized and reduced elements are both chlorine elements.
(2) Reactions involving elemental substances are not necessarily redox reactions. For example, the mutual transformation between allotropes (such as 3O2==discharge 2O3) is not a redox reaction.
use as you learn
1. True or false
(1) Oxygen must participate in the redox reaction. ()
(2) Oxidation-reduction reactions are characterized by changes in the valence of elements. ()
(3) When the metal element reacts with O2, the metal element undergoes an oxidation reaction. ()
(4) When Zn reacts with CuSO4 solution, Zn is reduced to Zn2+. ()
(5) In non-redox reactions, the valence of elements may change. ()
2. Which of the following reactions is a redox reaction? ()
A. CaCO3+2HCl===CaCl2+H2O+CO2↑
B. CaCO3=====High temperature CaO+CO2↑
C. Na2O+H2O===2NaOH
D. Mg+2HCl===MgCl2+H2↑
3. Among the following change processes, which one is a reduction reaction ()
A. HCl→Cl2 B. Na→Na+
C. CO→CO2 D. Fe3+→Fe
Redox Reaction PPT, Part 3: Knowledge Point 2: The Essence of Redox Reaction
1. An exploration of the essence of redox reactions
(1) Take the reaction between sodium and chlorine as an example
2Na+Cl2=====ignite 2NaCl
①The sodium atom loses electrons, the valence is 1________, and a 2________ reaction occurs;
②The chlorine atom gains electrons, the valence is 3___________, and a 4___________ reaction occurs.
(2) Take the reaction between iron and CuSO4 solution as an example
Cu2++Fe===Cu+Fe2+
① The iron atom loses electrons, the valence is 5________, and a 6________ reaction occurs;
②The copper ion gains electrons, the valence is 7___________, and an 8___________ reaction occurs.
(3)Conclusion
①The characteristics of oxidation-reduction reaction are 9____________________________.
②The essence of redox reaction is 10__________.
2. How to represent electron transfer in redox reactions
(1) Double line bridge method: Indicates the transfer of electrons between atoms of the same element before and after reaction. For example:
(2) Single line bridge method: represents the transfer of electrons between reactants during the reaction. For example:
Troubleshooting
How to represent electron transfer in redox reactions
(1)Double-line bridge method
①Basic steps
②Precautions
a. The arrows and tails point to the same element whose valence changes;
b. Mark "gain" and "lost", and the number of electrons gained and lost is equal.
(2)Single line bridge method
①Basic steps
②Precautions
a. Single line bridges must be drawn in reactants;
b. The arrow points to the element that has gained electrons, and the tail of the arrow points to the element that has lost electrons;
c. Indicate the total number of electronic transfers, not "gain" or "loss".
special reminder
(1) The total number of electrons transferred in the redox reaction is the total number of electrons gained or the total number of electrons lost, not the sum of the two.
(2) When the double-line bridge method is used to standardize electron transfer, the number of electron transfers is expressed in the form of "ae-×b", a represents the number of electrons lost or gained by each atom, and b represents the number of atoms that undergo oxidation or reduction reactions.
use as you learn
1. The essence of redox reaction is ()
A. There is a change in the valence of an element
B. gain and loss of oxygen atoms
C. electron transfer
D. New substances are produced after the reaction
2. Which of the following analysis of redox reactions is correct ()
3. (1) Analyze the chemical equations of the following reactions and determine the reaction type (redox reaction or non-redox reaction). If it is a redox reaction, use the double-line bridge method to mark the direction and number of electron transfer, and indicate the oxidized and reduced ones. element.
①2Na+Cl2====== Ignite 2NaCl
②NaOH+HCl===NaCl+H2O
Redox Reaction PPT, Part 4: Qualification Examination Training
1. Which of the following statements about redox reactions is correct ()
A. The valence of elements increases and decreases before and after the redox reaction
B. There must be gains and losses of oxygen before and after the redox reaction
C. The process of realizing the change of Fe3+→Fe2+ must be an oxidation reaction
D. Reactions that increase the valence of an element are reduction reactions
2. The reactions between the following groups of substances are both redox reactions and ionic reactions ()
A. Zinc reacts with dilute sulfuric acid
B. Reaction of hydrochloric acid and silver nitrate solution
C. Hydrogen burns in chlorine
D. Sodium oxide dissolves in water
3. (2019·Nanjing Zhonghua Middle School Senior Grade 1) The following reactions do not belong to the four basic reaction types, but they are oxidation-reduction reactions ()
A. 3CO+Fe2O3=====High temperature 2Fe+3CO2
B. Fe+CuSO4===FeSO4+Cu
C. AgNO3+NaCl===AgCl↓+NaNO3
D. 2KMnO4======△K2MnO4+MnO2+O2↑
4. The following change process can only be realized through oxidation reaction ()
A. HCl→H2 B. HCl→FeCl2
C. H2SO4(concentrated)→SO2 D. Fe→Fe2O3
Keywords: Free download of PPT courseware for compulsory course 1 of high school chemistry in Lu Ke version, PPT download of redox reaction, PPT download of elements and the world of matter, PPT download of understanding redox reaction, .PPT format;
For more information about the PPT courseware "Redox Reactions in the World of Elements and Matter, Understanding Redox Reactions", please click the "Redox Reactions in the World of Elements and Matter ppt, Understanding Redox Reactions ppt" tag.
"Integration of this Chapter" Elements and the Material World PPT:
"Integration of this Chapter" Elements and the World of Matter PPT Part 1 Content: Breakthrough Judgment of Large Coexistence of One Ion Example 1 A group of ions that can coexist in large quantities in both strongly acidic and strongly alkaline solutions is ( ) A.Na+, Cu2+, Cl -, SO_4^2- B.K+, Ca2+, NO_3^- ..
"Integration and Improvement at the End of Chapter" Elements and Material World PPT:
"End-of-Chapter Integration Improvement" Elements and Material World PPT Part One Contents: 1. Classification methods and their application in life 1. Classification of elements and substances (1) Elements exist in nature in free and combined states, and very active elements can only exist in combined states. like..
"Micro Project Scientific Use of Chlorine-Containing Disinfectants" Elements and the World of Matter PPT courseware:
"Micro Project Scientific Use of Chlorine-containing Disinfectants" Elements and the World of Matter PPT courseware Part One: Literacy Objectives 1. Predict the properties of chlorine-containing disinfectants from the perspective of substance categories and element valences. 2. Understand the common chlorine-containing disinfectants in daily life and use redox...
File Info
Update Time: 2024-11-25
This template belongs to Chemistry courseware Lu Ke Edition High School Chemistry Compulsory Course 1 industry PPT template
"Redox Reactions" Elements and the World of Matter PPT Courseware (Understanding Redox Reactions in Lesson 1) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Redox Reactions" Elements and the World of Matter PPT Courseware (Understanding Redox Reactions in Lesson 1) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Redox Reactions" Elements and the World of Matter PPT Courseware (Understanding Redox Reactions in Lesson 1), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview