People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| People's Education Press High School Chemistry Compulsory Course 2 | pptx | 6 MB |
Description
"Chemical Reactions and Energy Changes" Chemical Reactions and Energy PPT (Lesson 1 Chemical Reactions and Thermal Energy)
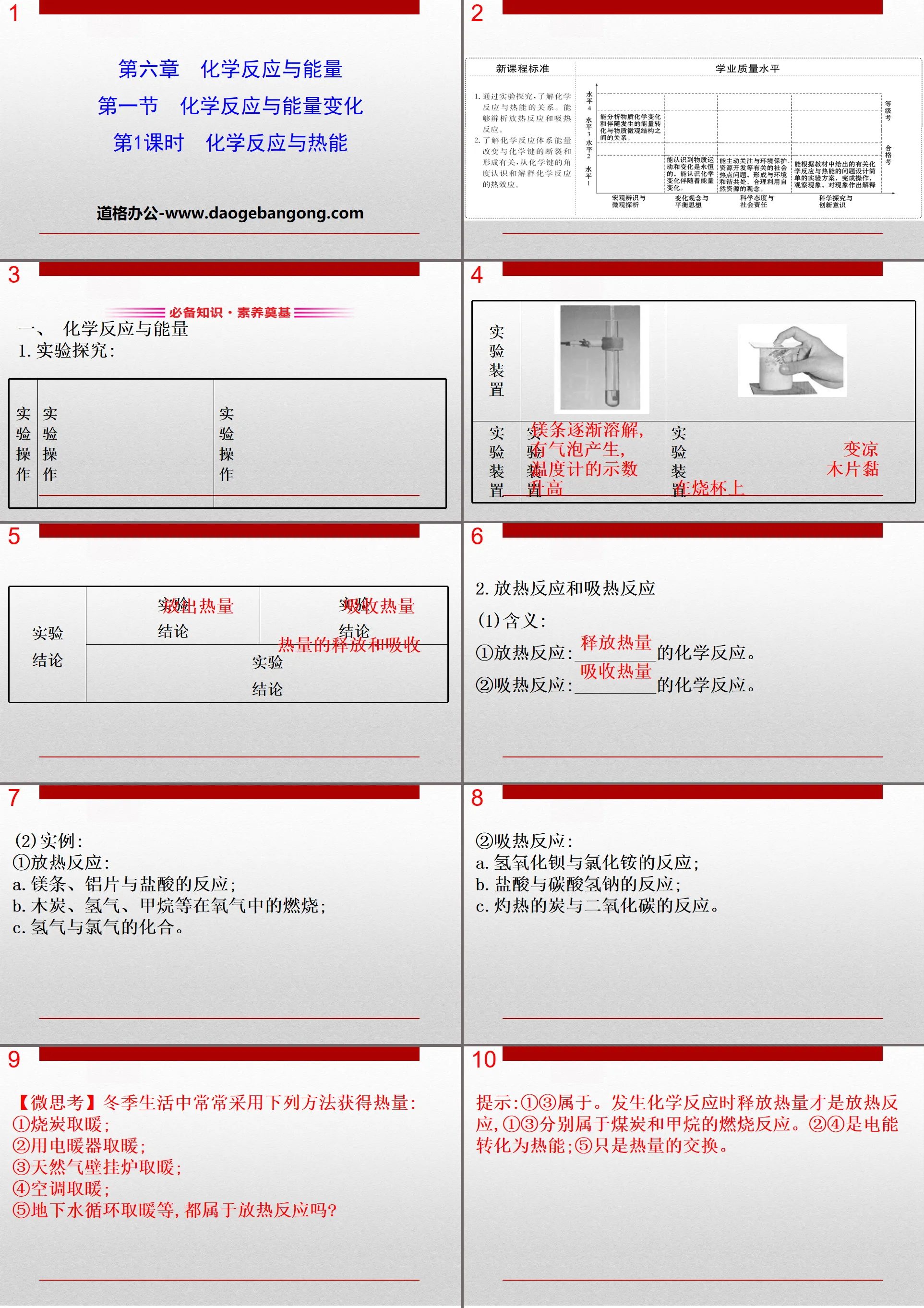
Part 1: Essential knowledge and foundation of literacy
1. Chemical reactions and energy
1.Experimental exploration:
2. Exothermic and endothermic reactions
(1)Meaning:
①Exothermic reaction: _________ chemical reaction.
② Endothermic reaction: _________ chemical reaction.
(2)Example:
①Exothermic reaction:
a. Reaction of magnesium strips, aluminum flakes and hydrochloric acid;
b. Combustion of charcoal, hydrogen, methane, etc. in oxygen;
c. The combination of hydrogen and chlorine.
② Endothermic reaction:
a. Reaction of barium hydroxide and ammonium chloride;
b. Reaction of hydrochloric acid and sodium bicarbonate;
c. The reaction between hot carbon and carbon dioxide.
[Micro Thoughts] The following methods are often used to obtain heat in winter life:
①Burning charcoal for heating;
②Use an electric heater to keep warm;
③Natural gas wall-mounted boiler for heating;
④Air conditioning and heating;
⑤ Are underground water circulation, heating, etc. all considered exothermic reactions?
Tips: ①③ belongs to. The release of heat when a chemical reaction occurs is an exothermic reaction. ① and ③ belong to the combustion reactions of coal and methane respectively. ②④ is the conversion of electrical energy into heat energy; ⑤ is just the exchange of heat.
2. The relationship between chemical bonds and energy changes in chemical reactions
1. The essential reasons for energy changes in chemical reactions:
(1) Reason:
(2)Example:
2. Analyze the energy changes of chemical reactions from multiple angles
【Smart Judgment】
(1) Endothermic reactions require heating to react. ()
Tip:×. For example, the reaction between barium hydroxide and ammonium chloride is endothermic and can proceed at room temperature.
(2) It is known that the reaction between Al and dilute hydrochloric acid is an exothermic reaction, that is, the energy of Al is greater than the energy of H2. ()
Tip:×. The heat release of the reaction shows that the total energy of the reactants is greater than the total energy of the products, that is, the total energy of Al and dilute hydrochloric acid is greater than the total energy of AlCl3 and H2, but the energies of Al and H2 are incomparable.
(3) Material changes that involve energy changes must be chemical changes. ()
Tip:×. Material changes that involve energy changes are not necessarily chemical changes. Physical changes in matter are also accompanied by energy changes. Such as ice melting, sodium hydroxide dissolving, etc.
3. Human use of energy
1. Three stages of utilization:
2. Two issues that need to be solved urgently during the utilization of fossil fuels
(1) First, it is non-renewable in the short term and has limited reserves;
(2) Second, dust, SO2, NOx, CO, etc. emitted by the combustion of coal and petroleum products are the main sources of atmospheric pollutants.
3. In the process of fuel utilization, the main aspects of energy saving are:
(1) Fuel combustion stage - the fuel combustion efficiency can be improved by improving the furnace type and fuel-air ratio of the boiler, cleaning up dust, etc.;
(2) Energy utilization stage - Energy recycling can be promoted through the use of energy-saving lamps, improvements in the materials and structures of electric motors, and cogeneration of waste heat from power plants and steel plants with urban heating, effectively improving energy utilization.
4. New energy
(1)Features: Rich resources, __________________.
(2) New energy sources that people are more concerned about: _____ energy, ___ energy, geothermal energy, ocean energy and ___ energy, etc.
[Situation·Thinking] The 2019 China (Shanxi) International Clean Energy Expo opened in Taiyuan on the morning of June 28, 2019.
With the theme of "Clean, Low-Carbon, Green Development", this exhibition focuses on the clean utilization of coal, new energy, energy storage, energy conservation and environmental protection, displays new technologies, new products and new applications, builds a supply and demand platform for clean energy and equipment in the Midwest, and promotes The implementation of coal-to-electricity, coal-to-gas, and coal-to-clean heating in the central and western regions will strengthen the control of smog and effectively improve the respiratory environment.
(1) What aspects should be considered to improve fuel combustion efficiency?
Tips: Make the fuel fully contact with oxygen, such as crushing, liquefying and gasifying coal; introduce sufficient oxygen.
(2) The following energy sources are often used in daily production and life:
① Coal; ② Petroleum; ③ Natural gas; ④ Solar energy; ⑤ Hydro energy; ⑥ Hydrogen energy;
⑦ Nuclear energy; ⑧ Wind energy; ⑨ Geothermal energy, etc. What are the clean energy sources?
Tips: ④⑤⑥⑦⑧⑨. Clean energy, or green energy, refers to energy that does not emit pollutants and can be directly used for production and life. It includes nuclear energy and renewable energy. Renewable energy refers to energy whose raw materials can be regenerated, such as hydropower, wind power, solar energy, water energy, biomass (biogas), geothermal energy (including ground and water sources), tidal energy and other energy sources.
Chemical Reaction and Energy Change PPT, Part 2 Content: Key Competencies·Quality Formation
Knowledge point 1: Understanding of exothermic reactions and endothermic reactions
[Key points to clarify doubts]
1. Comparison of exothermic reactions and endothermic reactions:
2. Common exothermic reactions and endothermic reactions
3. Two basic laws followed by chemical reactions
(1) Law of conservation of mass: When substances in nature are transformed, the total mass remains unchanged.
(2) Law of conservation of energy: One form of energy can be converted into another form of energy, but the total energy contained in the system remains unchanged.
【Think·Discussion】
(1) When doing experiments, alcohol lamps are used for both endothermic and exothermic reactions. How to judge whether the reaction is an endothermic reaction or an exothermic reaction? What is the purpose of heating for an exothermic reaction?
Tip: The reaction that continues to heat with an alcohol lamp and stops when the alcohol lamp is removed is an endothermic reaction; the reaction that continues to react after starting to heat with an alcohol lamp and continues to react after removing the alcohol lamp is an exothermic reaction. The purpose of heating for exothermic reactions is generally to initiate a reaction or speed up the reaction rate.
[Motif follow-up question] (1) In item B above, are dilute sulfuric acid and concentrated sulfuric acid containing equal amounts of H2SO4 reacting with a sufficient amount of sodium hydroxide solution to release the same amount of heat?
Tip: Not equal. The heat released when concentrated sulfuric acid reacts with a sufficient amount of sodium hydroxide solution is not only the heat released by the neutralization reaction, but also the heat released by the dilution of concentrated sulfuric acid.
(2) From the perspective of material energy, explain the reason why the ammonia synthesis reaction in item D above is exothermic?
Tip: In the ammonia synthesis reaction, the total energy of N2 and H2 participating in the reaction is greater than the total energy of NH3 generated, so the reaction is exothermic.
[Regular Method] "Four" Methods for Judging Exothermic Reactions and Endothermic Reactions
(1) Judgment based on reaction conditions. Reactions that generally require continued heating are endothermic reactions.
(2) Judgment based on energy change image. If the total energy of the reactants is greater than the total energy of the products, the reaction is exothermic; otherwise, the reaction is endothermic.
(3) Judgment based on the energy data absorbed and released by bond breaking and bond forming. If the energy absorbed by the breaking of old bonds is greater than the energy released by the formation of new bonds, it is an endothermic reaction; otherwise, it is an exothermic reaction.
(4) Empirical judgment method: judge based on the types of common exothermic reactions and endothermic reactions.
【Migration·Application】
1. (2019 Handan Senior High School Test) Which of the following statements is correct ()
A. Any chemical reaction is accompanied by changes in energy
B.H2O(g)→H2O(l) This process releases a lot of heat, so the process is a chemical change
C. Changes in energy in chemical reactions are manifested as changes in heat
D. For the process shown in the figure, it is a process of absorbing energy.
[Analysis] Choose A. Any chemical reaction has energy changes, but the process with energy changes is not necessarily a chemical change, such as the three-state change of matter. Although there is an energy change, there is no breaking of old chemical bonds and the formation of new chemical bonds, so it is not a chemical change. , Item B is wrong; There are many forms of energy changes in chemical reactions. In addition to heat energy, there are also light energy, electrical energy, etc. Item C is wrong; It can be seen from the image that the total energy of the reactants in the process is greater than the total energy of the products. Energy, so energy is released during this process, item D is wrong.
【Migration·Application】
1. (2019·Changsha Grade 1 Test) SF6 gas has a history of a hundred years. It is an artificial inert gas synthesized by two French chemists, Moissan and Lebeau, in 1900. Sulfur hexafluoride has good electrical insulation properties and excellent arc extinguishing properties, and is internationally known as "gas insulated switchgear". There are only S-F bonds in the molecular structure of SF6. It is known that the energy absorbed by converting 1 mol S (solid) into gaseous sulfur atoms is 280 kJ, and the energy absorbed to break 1 mol F-F and S-F bonds is 160 kJ and 330 kJ respectively.
Then the energy change of S(s)+3F2(g)====SF6(g) (it is known that s is a solid and g is a gas) is ()
A. Release 1 780 kJ of energy
B. Release 1 220 kJ of energy
C. Release 450 kJ of energy
D. Absorb 430 kJ of energy
[Analysis] Choose B. According to the question, the total energy absorbed by 1 mol S(s) and 3 mol F2 to form gaseous sulfur atoms and fluorine atoms = 280 kJ + 3 × 160 kJ = 760 kJ, and to generate 1 mol SF6 gas, 6 mol S— F bond, the total energy released is: 6×330 kJ=1 980 kJ, so the total energy released by this reaction is 1 980 kJ-760 kJ=1 220 kJ.
Keywords: PPT courseware for high school chemistry compulsory course 2 from the People's Education Press is free to download, chemical reaction and energy change PPT download, chemical reaction and energy PPT download, chemical reaction and thermal energy PPT download, .PPT format;
For more information about the PPT courseware "Chemical Reactions and Energy Changes Chemical Reactions and Energy Chemical Reactions and Thermal Energy", please click the Chemical Reactions and Energy Changes ppt Chemical Reactions and Energy ppt Chemical Reactions and Thermal Energy ppt tag.
"Chemical Reactions and Energy Changes" Chemical Reactions and Energy PPT (Lesson 2 Chemical Reactions and Electric Energy):
"Chemical Reactions and Energy Changes" Chemical Reactions and Energy PPT (Lesson 2 Chemical Reactions and Electric Energy) Part One Content: Foundation of Essential Knowledge Literacy 1. Conversion of chemical energy into electrical energy 1. Indirect conversion of chemical energy into electrical energy (2) Key combustion (Redox reactions). 【..
File Info
Update Time: 2024-10-01
This template belongs to Chemistry courseware People's Education Press High School Chemistry Compulsory Course 2 industry PPT template
"Chemical Reactions and Energy Changes" Chemical Reactions and Energy PPT (Lesson 1 Chemical Reactions and Thermal Energy) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Chemical Reactions and Energy Changes" Chemical Reactions and Energy PPT (Lesson 1 Chemical Reactions and Thermal Energy) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Chemical Reactions and Energy Changes" Chemical Reactions and Energy PPT (Lesson 1 Chemical Reactions and Thermal Energy), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview