People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Lu Ke Edition High School Chemistry Compulsory Course 2 | pptx | 6 MB |
Description
"Chemical Reactions and Energy Transformation" Chemical Bonding and Chemical Reaction Laws PPT (Lesson 1)
Part 1: Essential knowledge and foundation of literacy
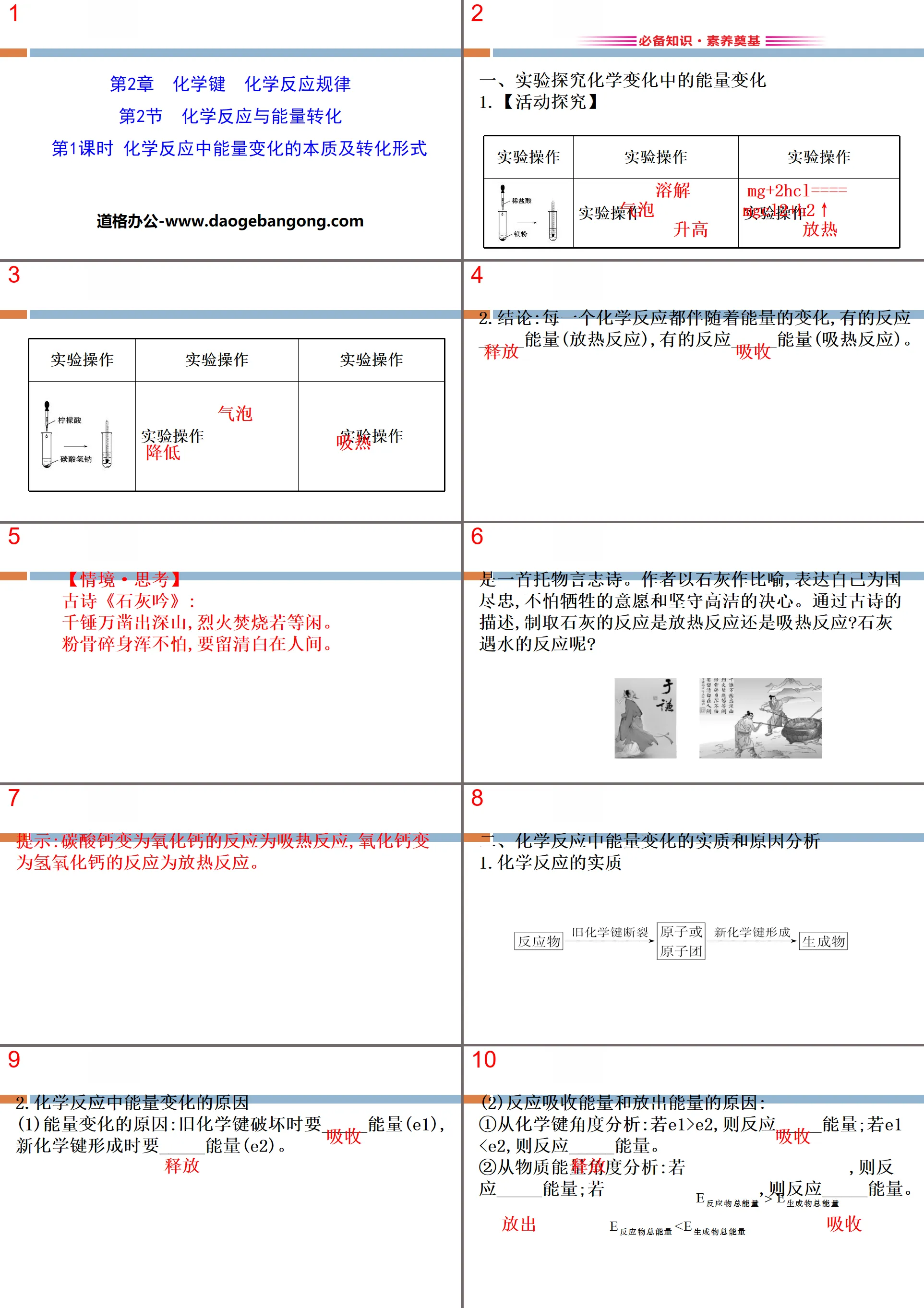
1. Experimental exploration of energy changes in chemical changes
1.【Activity Research】
2. Conclusion: Every chemical reaction is accompanied by changes in energy. Some reactions
_____ energy (exothermic reaction), some reactions _____ energy (endothermic reaction).
【Situation·Thinking】
Ancient poem "Song of Lime":
Thousands of hammers were used to carve out the deep mountains, and the fire burned them as if nothing happened.
Don't be afraid of your bones being shattered into pieces, you want to leave your innocence in this world.
It is a poem that expresses one's ambitions. The author uses lime as a metaphor to express his loyalty to the country, his willingness to not be afraid of sacrifice, and his determination to uphold integrity. According to the description in ancient poems, is the reaction of making lime an exothermic reaction or an endothermic reaction? What about the reaction of lime when it meets water?
Tip: The reaction of calcium carbonate to calcium oxide is an endothermic reaction, and the reaction of calcium oxide to calcium hydroxide is an exothermic reaction.
2. Analysis of the essence and causes of energy changes in chemical reactions
1.The essence of chemical reaction
2. Causes of energy changes in chemical reactions
(1) Reasons for energy changes: When old chemical bonds are broken, _____ energy is required (E1),
It takes _____ energy (E2) to form new chemical bonds.
(2) Reasons why reactions absorb energy and release energy:
①Analysis from the perspective of chemical bonding: If E1>E2, then the reaction energy is _____; if E1
②Analysis from the perspective of material energy: If _____, then the opposite Should _____ energy; if _____, then the reaction _____ energy. 【Micro thinking】 Does an exothermic reaction occur without heating? An endothermic reaction requires heating to occur? Hint: No. Whether the reaction is an exothermic reaction or an endothermic reaction has nothing to do with the reaction conditions. For example, the combustion of coal is an exothermic reaction, but it generally requires heating to occur; the reaction between ammonium chloride and calcium hydroxide is an endothermic reaction, but it can occur without heating. occur. Chemical Reaction and Energy Conversion PPT, Part 2: Key Competencies·Quality Formation Knowledge point 1: Causes and calculations of energy changes in chemical reactions [Key points to clarify doubts] 1. Analysis of energy changes in chemical reactions: (1) From the perspective of chemical bonding: ①E1>E2: The reaction absorbs energy (endothermic reaction). ②E1 It can be summarized as if the new is greater than the old, the reaction is exothermic; if the old is greater than the new, the reaction is endothermic. (2) From the perspective of energy changes within matter - "two processes": ① It can be regarded as the process in which energy external to matter, such as thermal energy, electrical energy or light energy, is "stored". ② It can be regarded as the process in which the energy (chemical energy) "stored" inside the substance is converted into heat energy, light energy or electrical energy and released. 2. Steps of energy calculation in chemical reactions (1) Determine the amount of bond-breaking and bond-forming substances according to the chemical equation. (2) Determine the total energy absorbed by bond breaking and the total energy released by bond forming. (3) Calculate the energy change of the reaction: ①If the reaction releases energy. E = sum of energy released when bonds are formed - sum of energy absorbed when bonds are broken ② If the reaction absorbs energy. E = sum of energy absorbed when bonds are broken - sum of energy released when bonds are formed [Error-prone reminder] When analyzing energy changes in chemical reactions, you should pay attention to the following two points: (1) The lower the energy content of a substance, the more stable it is. The energy change in a chemical reaction is related to the breaking and forming of chemical bonds. The energy absorbed by a broken chemical bond is equal to the energy released when the chemical bond is formed. The greater the energy value, the stronger the chemical bond, the more stable the substance itself, and the lower the energy of the substance. (2) Since there are breaks and formations of chemical bonds in any chemical reaction, any chemical reaction must also change energy when the material changes. That is to say, the total energy of the reactants in any chemical reaction must not be equal to the total energy of the products. Knowledge point 2: Comparison of exothermic reactions and endothermic reactions [Key points to clarify doubts] Comparison of exothermic and endothermic reactions [Error-prone reminder] Energy changes in chemical reactions are “not necessarily” (1) Reactions that require heating are not necessarily endothermic reactions, such as the reaction between carbon and oxygen. (2) Exothermic reactions may not necessarily occur easily at room temperature, such as thermite reaction. (3) Endothermic reactions do not necessarily require heating, such as the reaction between Ba(OH)2·8H2O crystals and NH4Cl crystals. (4) The exothermic (or endothermic) process is not necessarily an exothermic (or endothermic) reaction. For example, the melting of ice is an endothermic process rather than an endothermic reaction. 【Think·Discussion】 The picture is a schematic diagram of a disposable heating cup. When the water bag breaks, the water mixes with the solid pieces, and the temperature of the food in the cup gradually rises. What solid pieces can be used to make this heating cup? Tip: Quicklime is generally used for substances that release heat when exposed to water. 【Case Demonstration】 [Typical example] Use known information to determine whether the following reaction is an endothermic or exothermic reaction: (1) Determine whether the following reaction is an endothermic or exothermic reaction based on the changes in energy of the reactants and products: (2) Determine whether the reaction H2+Cl2 2HCl is an exothermic or endothermic reaction through calculation. (3) Determine whether the reaction is an endothermic or exothermic reaction through experimental phenomena as shown in the figure. Phenomenon: After the reaction, it was found that the glass piece stained with water and the beaker were frozen together. (4) Based on common exothermic reactions and endothermic reactions, determine which of the following reactions are exothermic reactions: ① Reaction between Al and Fe2O3 ② Reaction between Mg and CH3COOH solution ③ Combustion reaction ④ Neutralization reaction ⑤ Metathesis reaction Keywords: Free download of the PPT courseware of Lu Ke Edition High School Chemistry Compulsory Course Two, chemical reaction and energy conversion PPT download, chemical bond chemical reaction rules PPT download, the nature of energy changes in chemical reactions and transformation forms PPT download, .PPT format; For more information about the PPT courseware "Chemical Bonding Chemical Reaction Laws Chemical Reactions and Energy Conversion The Nature and Transformation Forms of Energy Changes in Chemical Reactions", please click on the Chemical Bonding Chemical Reaction Laws ppt Chemical Reactions and Energy Conversion ppt The Nature and Transformation Forms of Energy Changes in Chemical Reactions ppt label.
File Info
Update Time: 2024-11-22
This template belongs to Chemistry courseware Lu Ke Edition High School Chemistry Compulsory Course 2 industry PPT template
"Chemical Reactions and Energy Transformation" Chemical Bonding and Chemical Reaction Laws PPT (Lesson 1) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Chemical Reactions and Energy Transformation" Chemical Bonding and Chemical Reaction Laws PPT (Lesson 1) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Chemical Reactions and Energy Transformation" Chemical Bonding and Chemical Reaction Laws PPT (Lesson 1), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview