Fifth Grade Science Volume 1, Textbook Edition
Science Edition for Sixth Grade Science Volume 2
Science Edition for Sixth Grade Science Volume 1
Third Grade Science Volume 2, Textbook Edition
Fourth Grade Science Volume 2, Textbook Edition
Fourth Grade Science Volume 1, Textbook Edition
Third Grade Science Volume 1, Textbook Edition
Fourth-grade science volume 2 of the E-education edition
Qingdao Edition Fourth Grade Science Volume 2
E-education edition fifth grade science volume 1
Hunan Education Edition Fourth Grade Science Volume 1
E-education edition fifth grade science volume 2
Fifth Grade Science Volume 2, Textbook Edition
E-education edition sixth grade science volume 1
Zhejiang Education Edition Seventh Grade Science Volume 2
People's Education Press Fourth Grade Science Volume 2

| Category | Format | Size |
|---|---|---|
| Zhejiang Education Edition Seventh Grade Science Volume 1 | pptx | 6 MB |
Description
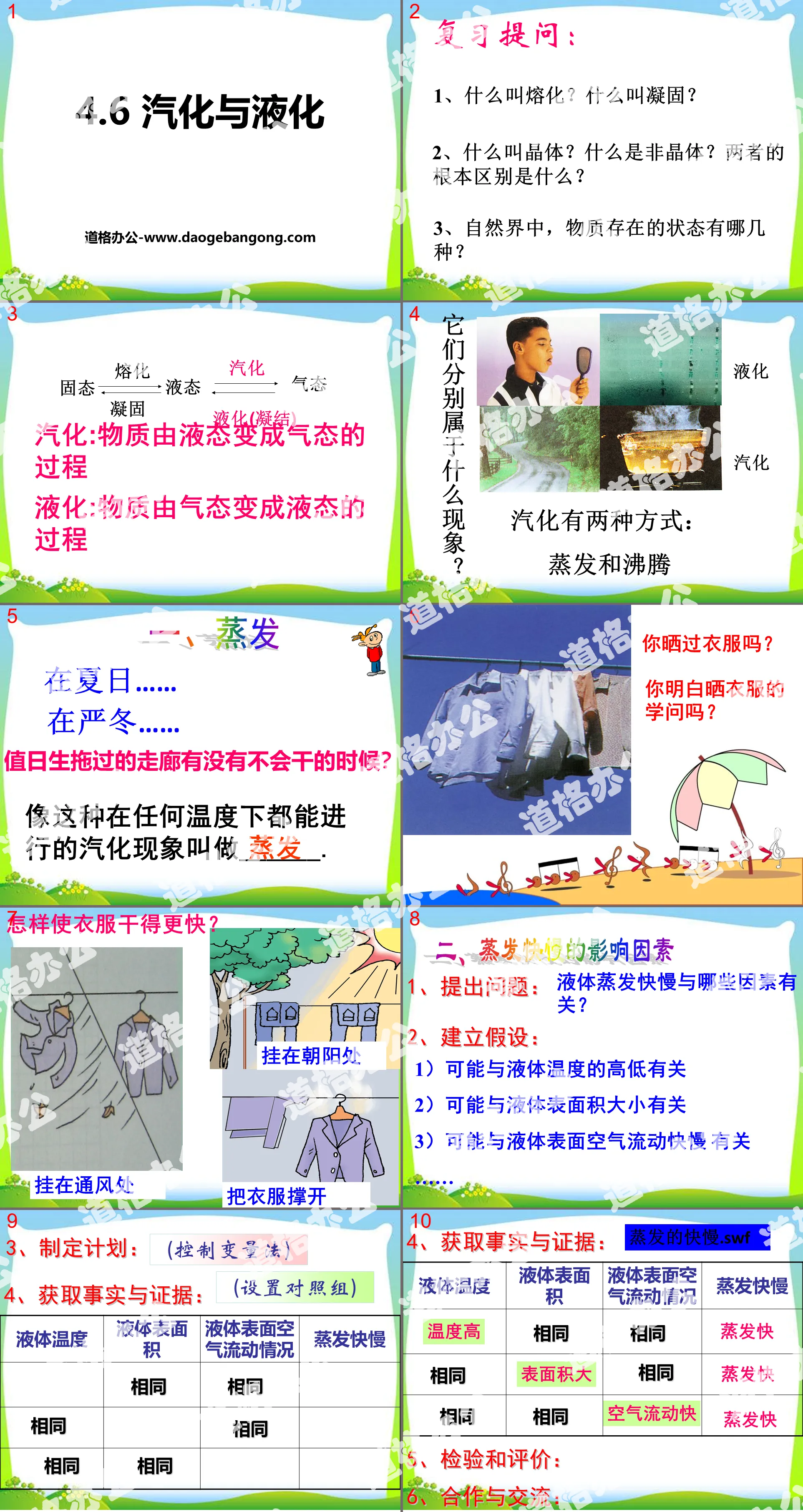
"Vaporization and Liquefaction" PPT
Part One: Review Questions:
1. What is melting? What is solidification?
2. What is a crystal? What is amorphous? What is the fundamental difference between the two?
3. What are the states of existence of matter in nature?
Vaporization and liquefaction PPT, Part 2: Learning about evaporation
1. Evaporation
In summer...
In the severe winter...
Is there ever a time when the corridor that the student on duty has mopped becomes dry?
This vaporization phenomenon that can occur at any temperature is called ______.
2. Factors affecting evaporation speed
1. Ask a question: What factors are related to the speed of liquid evaporation?
2. Establish hypotheses:
1) It may be related to the temperature of the liquid
2) It may be related to the size of the liquid surface area
3) It may be related to the speed of air flow on the liquid surface
3. Make a plan: (control variable method)
Vaporization and liquefaction PPT, part three: solid foundation
1. Wet clothes will dry out after a period of time. This is actually the ______ process of water. It is a kind of ______ phenomenon in the change of physical state. It can be carried out at ______ temperature.
2. When people come out of the swimming pool and are blown by the wind, they feel very cool. This is due to ___________. A glass of alcohol at 40℃ will continue to evaporate when the lid is opened, and the remaining alcohol will have a temperature of ______40℃ (fill in: above, below or equal to).
3. Fans make you feel cool in summer. This is because ( )
A. The wind blowing from the fan is cool
B. The wind blew away the heat from the body
C. The wind from the fan can speed up the evaporation of sweat and accelerate the absorption of heat from the human body.
D. Fans can lower the air temperature
Vaporization and liquefaction PPT, Part 4: Apply what you learn
Residents living in the African desert cannot use refrigerators to keep food fresh in the summer because there is no electricity. A junior high school student invented a "desert refrigerator" can-in-can, which consists of an inner can and an outer can. Moist sand is filled between the two cans. When used, food and drinks are placed in the inner can and the mouth of the can is opened. Cover with a damp cloth and place in a dry and ventilated place. Sprinkle some water on the sand between the two jars frequently to keep it fresh.
(1) The reason why we often sprinkle some water between the two cans is ____________________
(2) Place it in a dry and ventilated place to ____________________
Vaporization and liquefaction PPT, Part 5: Learning about boiling
boiling
1.The temperature change before water boils is ____________
2. After the water boils and continues to be heated, the internal phenomenon is ____________________
3.The change in temperature when water boils is_____________
If you stop heating the water after it boils, will the water continue to boil?
Characteristics of boiling
(1) It is a violent vaporization phenomenon
(2) Occurs simultaneously inside and on the surface of the liquid
(3) The boiling process must absorb heat and keep the temperature constant.
boiling point
Boiling point: The temperature at which a liquid boils
The relationship between boiling point and liquid surface pressure: the greater the liquid surface pressure, the higher the boiling point of the liquid
Boiling occurs at the boiling point. Below this temperature, the liquid absorbs heat and heats up, but does not boil. At this temperature, the liquid absorbs heat and boils, but does not heat up.
Vaporization and liquefaction PPT, Part 6: Can you explain the following phenomena?
(1) When people who wear glasses in winter go from cold outdoors to warm indoors, their lenses will be covered with a layer of small water droplets.
In real life, what other phenomena of "white gas" or "sweating" have been seen?
(2) When eating popsicles in the summer, the popsicles will emit white gas after removing the wrapping paper.
(3) When you open the refrigerator door, you can see a cloud of white gas.
(4) The outer wall of the cup filled with ice water will "sweat"
(5) The floors and furniture in some rooms will "sweat" during the rainy season.
Under what conditions can gas liquefy?
How gas is liquefied:
1. Cool down (gas will liquefy when it drops to a certain temperature)
Application: Helps separate mixed gases.
The temperature required for a gas to liquefy is called the liquefaction point. At 1 standard atmosphere, the liquefaction point of a gas is equal to the boiling point of its liquid state.
2. Pressurize and reduce the volume (compressed volume)
Application: Easy to transport and store.
Vaporization and liquefaction PPT, Part 7: Discussion
1. When the water in the kettle boils, why can't the "white gas" be seen in the section near the spout, but can be seen in the section above?
The temperature near the spout is higher and the water vapor is not liquefied, while the temperature of the water vapor in the back section is lower and liquefies into small water droplets, so it can be seen.
2. On winter nights, water droplets form on the window glass. Why?
Please think about the inside or outside of the glass?
There are water drops on the inside
In winter, when the room is hot inside and the outside is cold, the hot air in the room releases heat and liquefies when it encounters the cold glass, turning into small water droplets.
3. Being burned by 100℃ water vapor is much more serious than being burned by 100℃ water. Why?
Because water vapor needs to release heat to liquefy.
Vaporization and liquefaction PPT, Part 8: Practice
1. Today’s temperature is 20℃. Please judge the comparison between the readings of the following four thermometers and the temperature.
2. The following table lists the boiling points of three gases at standard atmospheric pressure. Now, if the mixed gas of these three gases is separated by liquefying it and then gradually raising the temperature, the order of the gases obtained is ( )
A. Oxygen, hydrogen, nitrogen B. Hydrogen, oxygen, nitrogen
C. Hydrogen, nitrogen, oxygen D. Oxygen, nitrogen, hydrogen
3. Covering fields with plastic film is a new technology in agricultural production - mulching film. The main function of mulching film is: __________________.
4. When we open the refrigerator door, we can see "white gas" coming out, which is formed when _________ water vapor encounters cold______.
5. In summer, classmate Xiaogang bought a watermelon, ate part of it and kept the rest. Among the following measures, the one that cannot prevent the water from evaporating is ( )
A. Put the watermelon in the refrigerator
B. Put the watermelon into the pressure cooker and seal it
C. Wrap the watermelon in plastic wrap
D. Cut the watermelon into small pieces and store it
Keywords: Zhejiang Education Edition seventh grade science PPT courseware free download, vaporization and liquefaction PPT download, .PPT format;
For more information about the "Vaporization and Liquefaction" PPT courseware, please click on the Vaporization and Liquefaction ppt tab.
"Vaporization and Liquefaction" PPT courseware:
"Vaporization and Liquefaction" PPT courseware Part 1: Exploring the boiling experiment of water 1. Ask questions 1. What are the characteristics of water when it boils? 2. What conditions are needed for water to boil? 3. If the water continues to be heated after it boils, will the temperature get higher and higher? 2. Guess (..
"Vaporization and Liquefaction" Temperature and Physical State Changes PPT Courseware 2:
"Vaporization and Liquefaction" Temperature and Physical State Changes PPT Courseware 2 Can liquid and gaseous states be transformed into each other? Experiment 1: Drop alcohol into a balloon or plastic bag, squeeze out the air and tie it tightly. Place in hot water. Please observe the phenomenon. 1. Vaporization and liquefaction Substances come from...
"Vaporization and Liquefaction" Temperature and Physical State Changes PPT Courseware:
"Vaporization and Liquefaction" Temperature and Physical State Changes PPT Courseware Learning Objectives: 1. Master the definition of vaporization, know the two ways of vaporization 2. Understand the phenomenon of evaporation, know that evaporation requires heat absorption and refrigeration, and be able to master the influencing factors of evaporation 3. Understand liquid boiling..
File Info
Update Time: 2024-11-23
This template belongs to science courseware Zhejiang Education Edition Seventh Grade Science Volume 1 industry PPT template
"Vaporization and Liquefaction" PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Vaporization and Liquefaction" PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Vaporization and Liquefaction" PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview