| Category | Format | Size |
|---|---|---|
| Lu Jiao Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
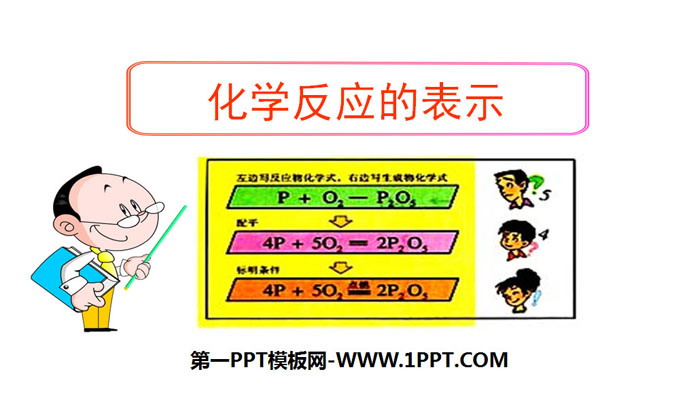
"Representation of Chemical Reactions" Quantitative Study of Chemical Reactions PPT, 24 pages in total.
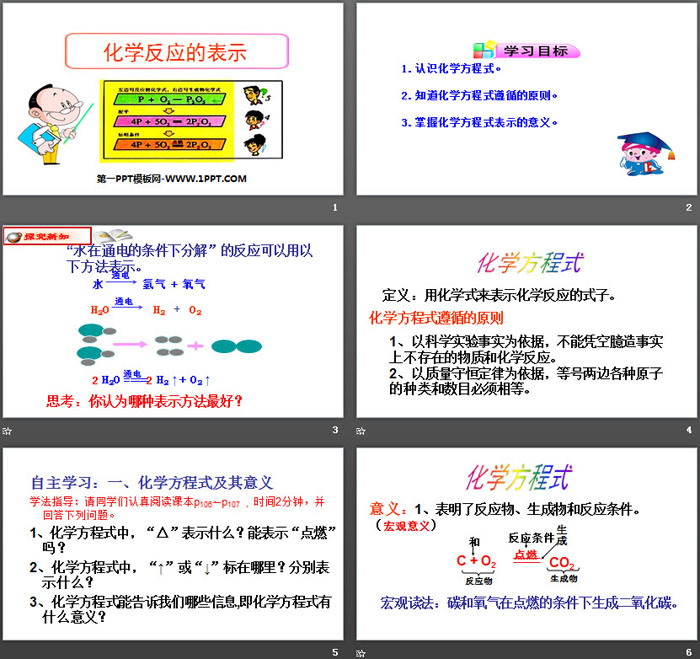
learning target
1. Understand chemical equations.
2. Know the principles followed by chemical equations.
3. Understand the meaning of chemical equations.
Explore new knowledge
The reaction "water decomposes under the condition of electricity" can be expressed in the following way.
chemical equation
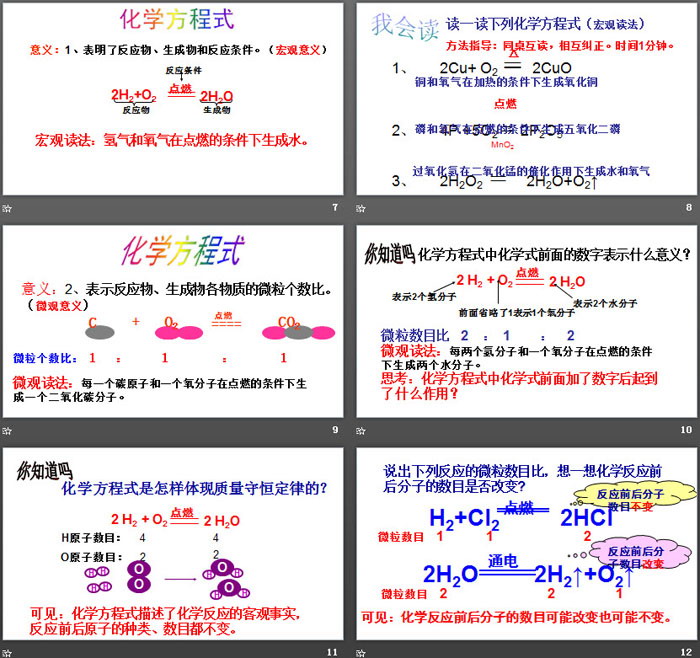
Definition: A chemical formula is used to represent a chemical reaction.
Principles followed by chemical equations
1. Based on scientific experimental facts, substances and chemical reactions that do not exist in fact cannot be invented out of thin air.
2. Based on the law of conservation of mass, the types and numbers of various atoms on both sides of the equal sign must be equal.
Independent learning: 1. Chemical equations and their significance
Study guide: Please read pages 106 to 107 of the textbook carefully for 2 minutes, and answer the following questions.
1. What does “△” represent in chemical equations? Can it mean "ignite"?
2. In the chemical equation, where is the mark "↑" or "↓"? What do they mean?
3. What information can chemical equations tell us, that is, what is the significance of chemical equations?
summary
1. Chemical equation
1. Definition
2. Follow the principles
⑴Follow objective facts ⑵Follow the law of conservation of mass
3. Meaning
⑴ Indicates the reactants, products and reaction conditions. (macro)
⑵Represents the ratio of the number of microscopic particles of reactants and products (microscopic)
⑶ Indicates the mass relationship between reactants and products, that is, the mass ratio between substances. (quality)
practice
1. Rockets use hydrazine (N2H4) as fuel and nitric oxide as oxidizer. The chemical equation of the reaction is: N2H4+2NO == 2X+2H2O. Find the chemical formula of X ( )
A. N2 B. NO2 C. NH3D. N2O
2. A certain substance can undergo the following reaction: 2 + 2H2O ==4KOH + O2↑, then the substance in should be ( )
A. KO B. K2O C. K2O2 D. KO2
Keywords: Free download of PPT courseware for the eighth grade of Chemistry in the May 4th System of Lujiao Edition, PPT download of the representation of chemical reactions, PPT download of quantitative research on chemical reactions, .PPT format;
For more information about the "Quantitative Study of Chemical Reactions Representation of Chemical Reactions" PPT courseware, please click the Quantitative Study of Chemical Reactions PPT Representation of Chemical Reactions PPT tab.
"Representation of Chemical Reactions" Quantitative Study of Chemical Reactions PPT (Lesson 2):
"Representation of Chemical Reactions" Quantitative Study of Chemical Reactions PPT (Lesson 2), 22 pages in total. Famous teacher’s eye-catching 1. Principles for writing chemical equations (1) Based on objective facts. (2) Follow the law of conservation of mass to keep the type and number of atoms consistent before and after the reaction.
"Representation of Chemical Reactions" Quantitative Study of Chemical Reactions PPT (Lesson 1):
"Representation of Chemical Reactions" Quantitative Study of Chemical Reactions PPT (Lesson 1), 21 pages in total. Famous teacher’s eye-catching 1. The significance of chemical equations (1) Qualitative aspect: indicates the reactants, products, and reaction conditions. (2) Quantitative aspect ① Macroscopic: Indicates the quality of each substance..
File Info
Update Time: 2024-06-29
This template belongs to Chemistry courseware Lu Jiao Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Representation of Chemical Reactions" Quantitative Study of Chemical Reactions PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Representation of Chemical Reactions" Quantitative Study of Chemical Reactions PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Representation of Chemical Reactions" Quantitative Study of Chemical Reactions PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview