| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 2 | pptx | 6 MB |
Description
"Purification of coarse salt" PPT courseware
Separation and purification of mixtures
Think and communicate
Do you know what Shali gold mining is?
What methods and properties do gold diggers use to separate gold from sand?
Wash: Rinse with water
Taking advantage of the different densities of substances
Gravel, sand: Density=2.5~3.5g/cm3
Gold: Density=19.3g/cm3
The existence form of matter in nature
Pure substances (very few)
mixture (mostly)
Chemistry studies the properties of substances
It is the study of the properties of pure substances
Learning and inquiry
When separating and purifying substances, impurities are removed. Are all chemical impurities harmful and worthless? Can you give an example?
For example, there may be associated gold in copper mines. Gold is also an impurity for refining copper. For example, water containing some minerals and trace elements is beneficial to the human body. It is necessary to evaluate whether the impurity is harmful or valuable in human activities based on its nature and content.
What is the difference between isolation and purification?
Separation is to separate several substances in a mixture through appropriate methods and obtain pure substances respectively.
Purification is the removal of impurities from a mixture through appropriate methods to obtain pure substances.
Commonly used methods are: filtration, evaporation, distillation, and extraction.
Physical methods: dissolution, filtration, crystallization, gasification, evaporation, fractionation, extraction
Chemical methods: transformation method, (heating, precipitation, replacement)
Requirements for chemical purification:
1. The final result is that no new substances are introduced
2. Generally, the reagents added only react with impurities and turn into precipitation, gas or water.
3. The principle is correct, the operation is simple, the cost is not high, and no harmful substances are generated (preferably no waste is produced)
Please recall the method of purifying coarse salt
Coarse salt ingredients: NaCl [CaCl2, MgCl2, sulfate, insoluble impurities]
Operation process: Dissolution - Filtration - Evaporation
Filtration operation essentials: one stick, two low, three stick
Evaporation operation essentials: The glass rod should be stirred constantly to prevent local boiling; stop heating when there are more solids.
Experimental principle
Coarse salt contains insoluble impurities (sand, etc.) and soluble impurities (magnesium chloride, calcium chloride, etc.). In this experiment, through operations such as dissolving, filtering, and evaporating the filtrate of coarse salt, water-insoluble impurities such as sediment can be removed, and then the water is evaporated to obtain purer refined salt, thereby preliminary purifying the coarse salt.
Experimental drugs and instruments
Medicines: Coarse salt, water
Equipment: pallet balance, measuring cylinder, beaker, glass rod, medicine spoon, funnel, filter paper, iron stand (with iron ring), evaporating dish, alcohol lamp, crucible tongs, rubber dropper, matches
1. Purification of coarse salt
(1) Dissolve
Operation: Weigh about 4g of coarse salt and add it to a beaker containing about 12mL of water. Stir with a glass rod while adding until the coarse salt is no longer dissolved.
Phenomenon: The solid salt gradually dissolves and decreases, and the salt water becomes slightly turbid.
(2) Filter
Operation: Pour the liquid in the beaker into the filter along the glass rod. The liquid level of the filter should not exceed the edge of the filter paper. If the filtrate is turbid, filter it again
Phenomenon: Insoluble matter remains on the filter paper, the liquid seeps through the filter paper, and flows into another beaker along the funnel neck.
(3) Evaporation
Operation: Pour the filtrate into the evaporating dish, then heat it with an alcohol lamp, and at the same time stir the solution with a glass rod. Stop heating when more solids appear.
Phenomenon: water evaporates and solids gradually precipitate
3. Inspection of substances
1. Principle:
According to the physical properties of the substance (such as color, state, smell, density, etc.) or chemical properties (special phenomena such as gas generation, precipitation, etc.)
2. Note:
(1) Choose reasonable reagents (reagents that are simple to operate, sensitive to reaction, and have obvious phenomena)
(2) Reagents cannot be added directly to the substance to be tested, and samples must be taken; solids are generally prepared into solutions first.
(3) During operation, attention should be paid to the interference of certain common phenomena.
Testing methods for several common ions
1. Testing method for Cl-
First add dilute HNO3 to acidify, then add AgNO3 solution. If a white precipitate that is insoluble in dilute nitric acid appears, chloride ions are present.
When describing, it should be explained: what reagents were used + what phenomena occurred + what conclusions were drawn
2. Inspection method of SO42-
First add dilute HNO3 or dilute HCl to acidify, and then add soluble barium salt solution. If a white precipitate insoluble in dilute acid appears, then sulfate ions are present.
Pay attention to eliminate the interference of CO32- plasma. The choice of acid and barium salt should depend on the specific situation and cannot be static.
3. Testing of several other common ions
H+ Add purple litmus test solution to the solution to be tested. The solution turns red, which proves that the solution is acidic and H+ is present.
OH- Add phenolphthalein test solution to the solution to be tested. The solution turns red, which proves that the solution is alkaline and contains OH-
CO32- Add hydrochloric acid to the liquid to be tested, and the colorless and odorless gas produced will be passed into the clear lime water to produce turbidity, which proves the presence of CO32-
practise
1. In addition to soluble impurities such as calcium ions, magnesium ions, and sulfate ions, coarse table salt also contains insoluble impurities such as mud and sand. The refined salt we consume is obtained by purifying coarse table salt. Answer the following questions through the "Purification of Coarse Salt" in the textbook and the experiment you have done.
(1) When evaporating NaCl solution in the laboratory, the following procedures are generally followed: ① place an alcohol lamp; ② fix the position of the iron ring; ③ place the evaporating dish (the evaporating dish contains NaCl solution); ④ heat and stir; ⑤ stop heating. The correct sequence of operations is ____________.
(2) How to use the simplest method to check whether there are SO42- ions in the solution? __________. If so, how should SO42- ions be removed? _________.
(3) Add saturated Na2CO3 solution dropwise to the dissolved and filtered solution of crude salt until no more precipitation occurs. What is the purpose of this step?
(4) Filter the solution after operation (3). What impurities can be removed by this operation? _________.
(5) In the process of turning coarse salt into refined salt in the laboratory, glass rods are used in the three steps of dissolution, filtration, and evaporation. Explain the purpose of using glass rods in these three situations;
When dissolved: _________. When filtering: _________. When evaporating: _________.
2. In the "Crude Salt Purification" experiment, during evaporation, which of the following operations is correct ( ).
A. Pour the turbid liquid into an evaporating dish and heat it
B. Stir with a glass rod after crystals begin to precipitate.
C. Stop heating when a large amount of solid appears in the evaporating dish
D. Stop heating after the liquid evaporates.
3. The following instruments are used in the experiment of purifying coarse salt ( ).
① Crucible ② Evaporating dish ③ Beaker ④ Water tank ⑤ Test tube ⑥ Funnel
A.②③⑥ B.③⑤⑥ C.②③④ D.②③⑤⑥
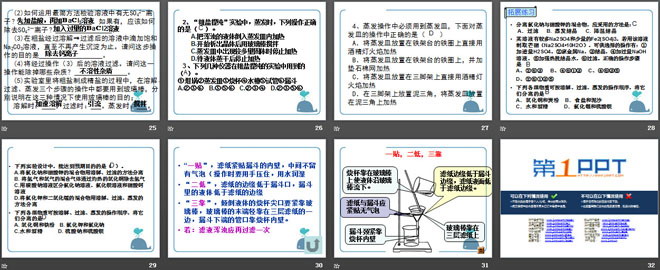
"One stick", the filter paper is close to the inner wall of the funnel, leaving no air bubbles in the middle (press it with your hands and moisten it with water during operation)
"Two low", the edge of the filter paper is lower than the mouth of the funnel, and the liquid in the funnel is lower than the edge of the filter paper
"Three supports": the tip of the beaker for pouring liquid should be close to the glass rod, the end of the glass rod should be lightly placed against one side of the three-layer filter paper, and the nozzle at the lower end of the funnel should be placed against the inner wall of the beaker.
If: the filtrate is turbid, it should be filtered again
Keywords: Purification of coarse salt teaching courseware, Hunan Education Edition ninth grade chemistry PPT courseware download, second volume, ninth grade chemistry slide courseware download, purification of coarse salt PPT courseware download, .PPT format;
For more information about the PPT courseware "Purification of Coarse Salt", please click on the PPT tab of "Purification of Coarse Salt".
"Purification of coarse salt" PPT courseware 2:
"Purification of Coarse Salt" PPT Courseware 2 Questions are allowed. Do you have any questions when you see the topic of substance purification? Students: 1) What is purification? 2) Why purify? 3) How to purify? 4) Purification equipment learned Purity of substances Pure substance: a substance, such as:..
File Info
Update Time: 2024-07-02
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 2 industry PPT template
"Purification of coarse salt" PPT courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Purification of coarse salt" PPT courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Purification of coarse salt" PPT courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview