| Category | Format | Size |
|---|---|---|
| People's Education Press High School Chemistry Compulsory Course I | pptx | 6 MB |
Description
"Classification of Substances" Classification and transformation of substances PPT courseware
Part 1: Essential knowledge and foundation of literacy
1. Classification according to the composition and properties of substances
1. Classification according to the composition of substances
(1) The basis of chemical research: Classifying substances according to _________ is
Fundamentals of chemical research.
(2) The form of matter composed of elements:
2. Common methods of classifying substances
(1) Tree classification method:
①Meaning: A classification method that reclassifies similar things according to certain attributes.
②Example: Substances can be classified as follows according to their composition:
【Do it】
Drugs in laboratories are often placed regularly according to the nature and category of substances. Medicines should be placed in designated places after use. When doing the "Properties of Acid" experiment, some medicines are placed on the experimental table as shown in the figure. Where should Xiao Ming put the potassium hydroxide solution back after taking it?
Tip:D. Potassium hydroxide is an alkali and should be placed at the position of the alkali, which is D.
(2) Cross classification method:
①Meaning: A classification method that classifies the same thing in multiple ways according to different classification standards.
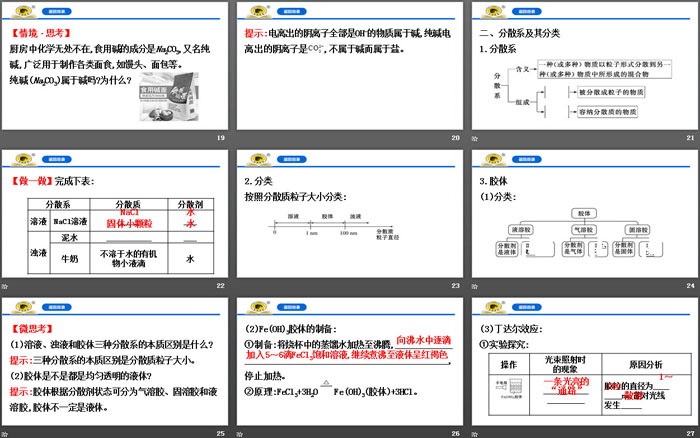
2. Dispersion systems and their classification
1. Dispersion system
2. Classification
Classification according to the size of dispersed particles:
3. Colloid
(1)Category:
【Micro thinking】
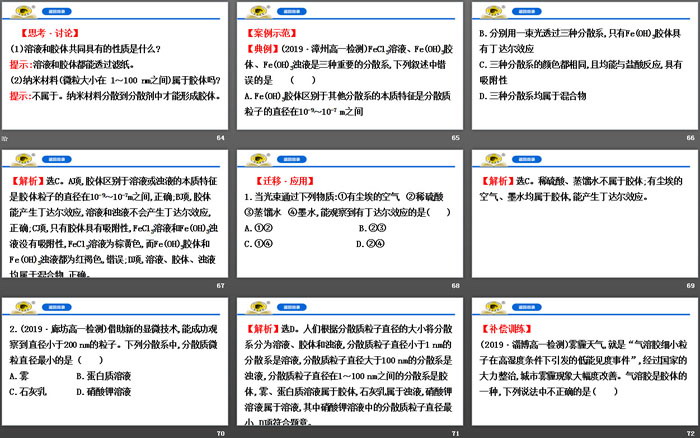
(1) What are the essential differences between the three dispersions of solution, turbid liquid and colloid?
Tip: The essential difference between the three dispersion systems is the size of the dispersed particles.
(2) Are colloids uniform and transparent liquids?
Tip: Colloids can be divided into aerosols, solid sols and liquid sols according to the state of the dispersant. Colloids are not necessarily liquids.
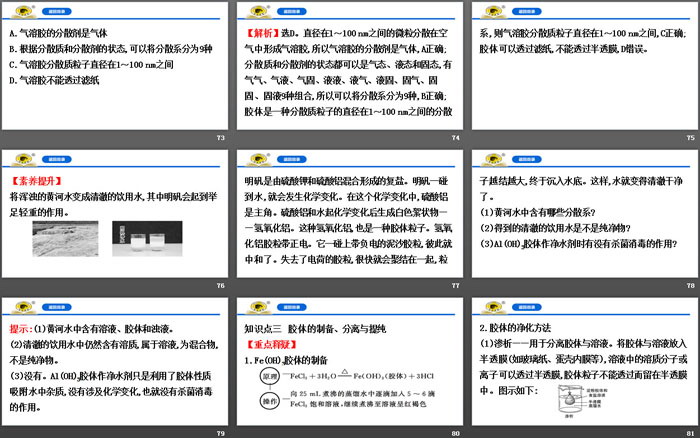
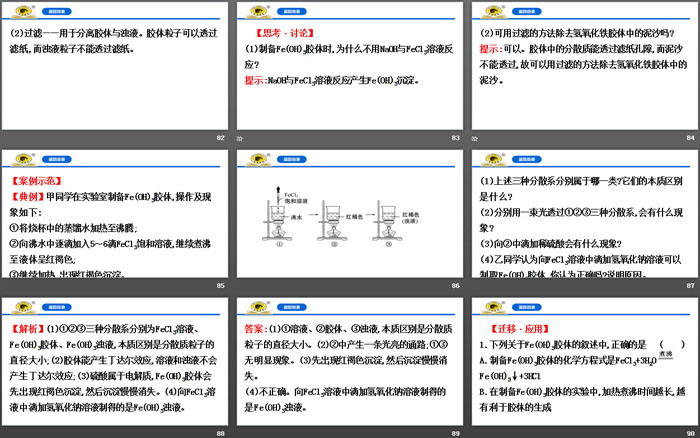
(2) Preparation of Fe(OH)3 colloid:
① Preparation: Heat the distilled water in the beaker to boiling, add 5 to 6 drops of FeCl3 saturated solution drop by drop to the boiling water, and continue to boil until the liquid turns reddish brown.
Stop heating.
②Principle: FeCl3+3H2O Fe(OH)3(colloid)+3HCl.
【Situation·Thinking】
The Tyndall effect can be observed when sunlight shines into a dark room from a window, or when light shines into a dense forest through gaps between leaves. When playing a movie, the formation of a light beam hitting the screen in the projection room also belongs to the Tyndall effect.
(1) Can all dispersion systems produce the Tyndall effect?
(2) How to quickly and easily distinguish solutions and colloids?
Tips: (1) Tyndall effect is a unique property of colloids;
(2) Using whether the Tyndall effect can occur can quickly and easily distinguish solutions and colloids.
Classification of substances PPT, part 2: key abilities and literacy formation
Knowledge point 1: Composition and classification of matter
[Key points to clarify doubts]
1. Characteristics and differences between the two classification methods
2. Classification of chemical substances
[Error-prone reminder]
(1) The number of acids is based on the number of H+ ionized by the acid, not the number of hydrogen atoms in the molecule. For example, CH3COOH is a monobasic acid rather than a tetrabasic acid.
(2) Two misunderstandings about pure substances and mixtures:
① Mistakenly believing that a mixed system composed of the same substance in different states is a mixture. Ice and water are in different states, but their components are both water, so the ice-water "mixture" is actually a pure substance.
② Mistakenly believing that substances composed of the same elements are pure substances. Diamond and graphite are both elements composed of carbon, but a mixture of the two is a mixture.
(3) Understanding of acidic oxides and basic oxides
①Acidic oxide
a. Acidic oxides are not necessarily non-metal oxides, such as Mn2O7.
b. Non-metal oxides are not necessarily acidic oxides, such as CO and NO.
②Alkaline oxide
a. Basic oxides must be metal oxides.
b. Metal oxides are not necessarily basic oxides, such as Mn2O7, which is an acidic oxide.
【Think·Discussion】
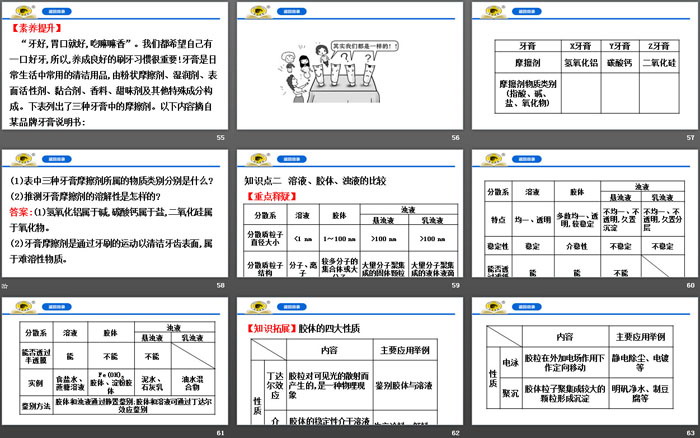
(1) Is the substance in the solution that is acidic an acid, and the substance that is alkaline in a solution a base? Give an example.
Hint: No. NaHSO4 solution is acidic and belongs to salts; Na2CO3 solution is alkaline and belongs to salts.
(2) What type of substance does the water purifying agent alum[KAl(SO4)2·12H2O] belong to?
Tip: Alum is a salt.
(3) Are salts necessarily compounds containing metal elements? Please give examples.
Tip: Not necessarily. For example, ammonium salt NH4NO3 is a compound composed entirely of non-metallic elements.
(4) Are there any oxides that are neither acidic oxides nor alkaline oxides? Please give examples.
Tip: Yes. Oxides such as CO and NO are non-salt-forming oxides.
【Migration·Application】
1. "Shun Dian" is appended with a preface to "Shu": "The emperor came down to earth, set up residences, and classified them separately, and wrote "Gun Zuo"." The classification method played an important role in the development of chemistry. The following classification is correct ()
[Analysis] Choose A. Item B, brass, hydrochloric acid, and limewater are mixtures, HCl and Ca(OH)2 are acids and bases respectively, which is incorrect; item C, iodine is a mixture, which is incorrect; item D, soda ash is a salt, which is incorrect.
2. In a broad sense, oxides refer to binary compounds formed by oxygen and another element. Which of the following statements is correct ()
A. Non-metal oxides must be acidic oxides
B. Acidic oxides must be non-metal oxides
C. Basic oxides must be metal oxides
D. Metal oxides must be alkaline oxides
[Analysis] Choose C. CO is not an acidic oxide, A is wrong; Mn2O7 is an acidic oxide, B is wrong; Na2O2 is a peroxide, D is wrong.
【Compensation training】
1. If a gas contains only one element after testing, then the gas is ()
A. A simple substance
B.a compound
C. Mixture of elements and compounds
D. It may be a single substance or a mixture of several simple substances.
[Analysis] Choose D. If it contains only one element, the substance may be a single substance or a mixture of several simple substances, such as a mixture of O2 and O3.
【Quality Improvement】
"If your teeth are good, your appetite will be good, and you will feel good when you eat it." We all want to have good teeth, so it is important to develop good brushing habits! Toothpaste is a commonly used cleaning product in daily life. It consists of powdery friction agent, wetting agent, surfactant, adhesive, spice, sweetening agent, etc. Agents and other special ingredients. The table below lists the abrasives found in three toothpastes. The following content is excerpted from the instruction manual of a certain brand of toothpaste:
Keywords: Free download of the PPT courseware for high school chemistry compulsory course 1 from the People's Education Press, PPT download of the classification of substances, PPT download of the classification and transformation of substances, .PPT format;
For more information about the PPT courseware "Classification of Substances, Classification and Transformation of Substances", please click on the Classification of Substances PPT Classification and Transformation of Substances PPT tag.
"Classification of Elements and Matter" Elements and the Material World PPT (Lesson 2: Colloids):
"Classification of Elements and Matter" Elements and the World of Matter PPT (Lesson 2: Colloids) Part One Content: Literacy Objectives 1. Through experimental verification and understanding of the classification of dispersions, know that colloids are a common dispersion system. 2. Understand the Tyndall discovery of colloids through experimental facts..
"Classification of Elements and Matter" Elements and the Material World PPT (Lesson 1: Relationship between Elements and Matter, Classification of Matter):
"Classification of Elements and Matter" Elements and the Material World PPT (Lesson 1: The Relationship between Elements and Matter, Classification of Matter) Part One Content: Literacy Goal 1. Pay attention to the elemental composition of matter, know that elements are components of matter, and elements are in the free state And the two forms of combined state..
"Classification of Elements and Matter" PPT courseware of elements and the world of matter (an important mixture in Lesson 2 - colloid):
"Classification of Elements and Substances" PPT courseware of elements and the world of matter (an important mixture of colloids in Lesson 2) Part One: Introduction of new lesson [Discussion and exploration] Understand the liquids in life: 1. Water 2. Alcohol 3. Sulfuric acid 4. Dilute sulfuric acid 5. Sea water 6. Chlorine..
File Info
Update Time: 2024-06-30
This template belongs to Chemistry courseware People's Education Press High School Chemistry Compulsory Course I industry PPT template
"Classification of Substances" Classification and transformation of substances PPT courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Classification of Substances" Classification and transformation of substances PPT courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Classification of Substances" Classification and transformation of substances PPT courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview