People's Education Edition Physics for Grade 8, Volume 2
People's Education Edition Physics for Grade 8, Volume 1
People's Education Edition Ninth Grade Physics Complete Book
Shanghai Science Edition Ninth Grade Physics
Shanghai Science Edition 8th Grade Physics
Beijing Normal University eighth grade physics volume one
Lu Jiao Edition Ninth Grade Physics Volume 2
Beijing Normal University Ninth Grade Physics Volume 1
Lu Ke Edition High School Physics Compulsory Course One
Guangdong and Shanghai Edition Ninth Grade Physics Volume 1
People's Education Press High School Physics Compulsory Course II
Lu Jiao Edition Ninth Grade Physics Volume 1
Beijing Normal University Ninth Grade Physics Volume 2
Lu Jiao Edition Eighth Grade Physics Volume 2
Lu Jiao edition eighth grade physics volume 1
Guangdong and Shanghai Edition Ninth Grade Physics Volume 2

| Category | Format | Size |
|---|---|---|
| Beijing Normal University Ninth Grade Physics Volume 1 | pptx | 6 MB |
Description
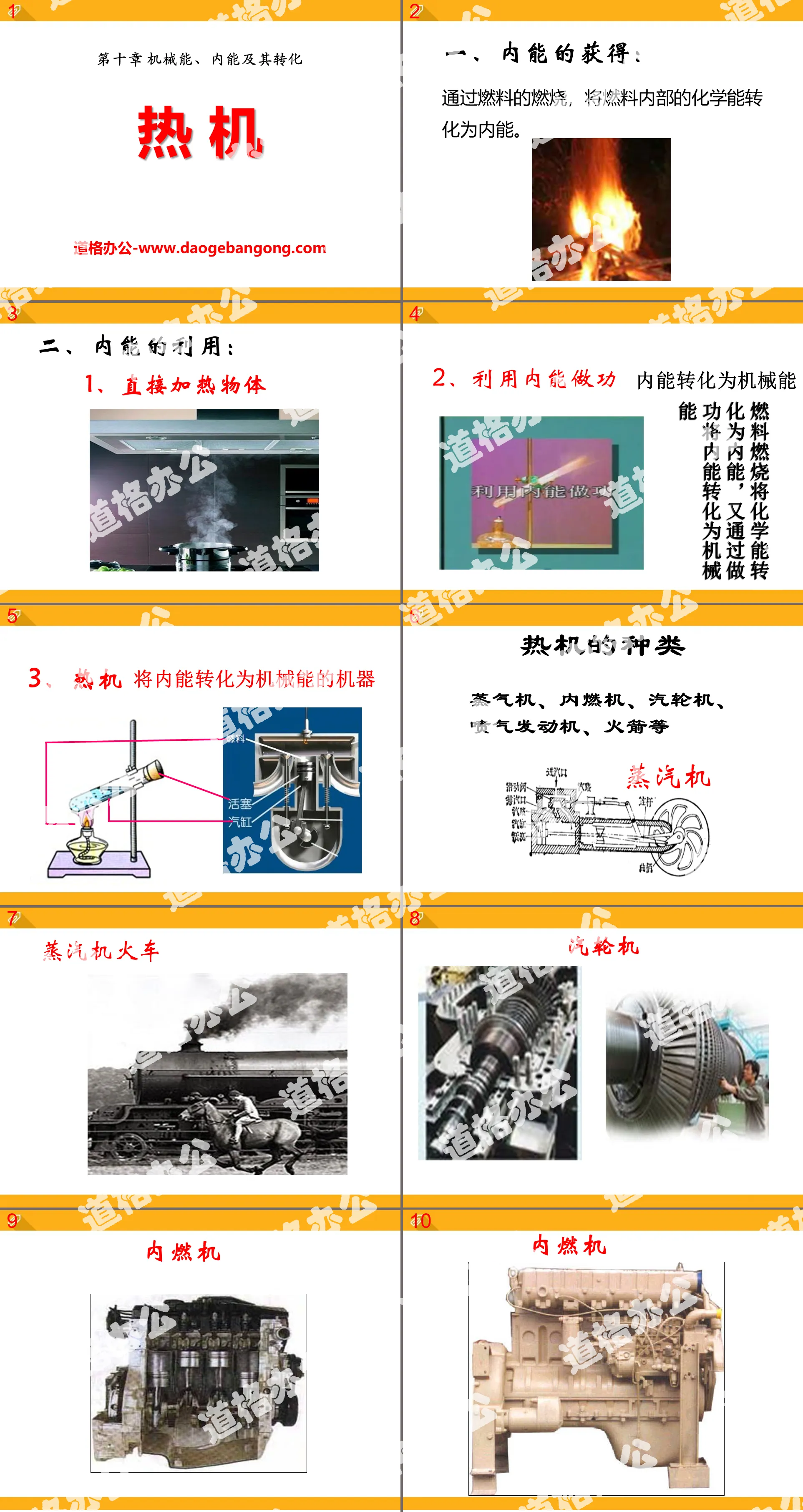
"Heat Engine" Mechanical energy, internal energy and their conversion PPT courseware 2
1. Acquisition of internal energy:
Through the combustion of fuel, the chemical energy inside the fuel is converted into internal energy.
2. Utilization of internal energy:
1. Directly heat objects
2. Use internal energy to do work
Internal energy converted into mechanical energy
Fuel combustion converts chemical energy into internal energy, and then converts internal energy into mechanical energy through work
3. Heat engine
A machine that converts internal energy into mechanical energy
Types of heat engines
Steam engine, internal combustion engine, steam turbine, jet engine, rocket, etc.
Want to discuss
1. Among the four strokes, which strokes undergo energy conversion?
2. Which stroke gives the car power?
3. Which stroke discharges the exhaust gas from the car?
Ignition method: compression ignition
At the end of the compression stroke, the mist diesel fuel sprayed from the fuel injector burns violently when it encounters hot air, producing high-temperature and high-pressure gas, which pushes the piston downward and drives the crankshaft to rotate through the connecting rod.
Ignition method: pilot type
At the end of the compression stroke, the spark plug generates an electric spark, which causes the fuel to burn violently, producing high-temperature and high-pressure gas, which pushes the piston downward and drives the crankshaft to rotate through the connecting rod.
3. Calorific value of fuel:
1. There are many types of fuels, which can be divided into solid fuels, liquid fuels and gaseous fuels.
2. Burning different fuels of the same mass releases different amounts of heat.
3. Calorific value of fuel: The heat released by the complete combustion of 1kg of a certain fuel is called the calorific value of the fuel.
4. Unit of calorific value: J/kg
5. The symbol of calorific value: q
4. Fuel utilization:
think:
In the above example, Xiaoming used 1.5kg of bituminous coal to boil water. The heat released by complete combustion of these coals is 4.05×107J. Is the actual heat released 4.05×107J? Why? What about the actual amount of heat absorbed by the water?
The heat actually absorbed by water is called effectively utilized heat.
1. Furnace efficiency:
The ratio of the heat effectively utilized (heat absorbed by water) to the heat released by complete combustion of the fuel.
Formula: η=Q suction/Q release=cm△t/mq
2. Improve fuel utilization and save energy
1) Make coal fully burn: Grind the coal into coal particles and increase the air supply volume.
2) Increase the heating area and reduce the heat taken away by the flue gas:
3. Efficiency of a heat engine: The ratio of the part of energy used to do useful work to the heat released when the fuel is completely burned is called the efficiency of the heat engine.
Incomplete combustion of fuel, heat loss after cylinder components absorb heat, heat taken away by exhaust gas, and energy consumed to overcome friction and perform work are all causes of energy loss. Therefore, improving the efficiency of heat engines can improve fuel utilization.
Ways to improve heat engine efficiency and energy saving methods:
(1) Reduce various heat losses as much as possible, such as ensuring good lubrication and reducing the energy consumed by overcoming friction;
(2) Make full use of the energy of waste gas and improve fuel utilization, such as using waste gas from thermal power stations to provide heat. This kind of thermal power station that provides both power and heat has much higher fuel utilization than ordinary thermal power stations.
calculate:
1. How much heat can be produced by completely burning 15 kilograms of charcoal with a calorific value of 3.4×107 Joules/kg?
2. How many kilograms of hydrogen need to be completely burned to obtain this amount of heat? (q hydrogen = 1.4 × 108 Joules/kg)
3. To boil 5kg of water at 40℃, how many kilograms of dry firewood need to be completely burned?
(It is known that the combustion value of dry wood is 1.2×107J/kg, the external pressure is standard atmospheric pressure, and all the heat released by complete combustion of the fuel is absorbed by water)
Keywords: Thermal engine teaching courseware, Mechanical energy internal energy and its conversion teaching courseware, Beijing Normal University edition ninth grade physics PPT courseware download, Ninth grade physics slide courseware download, Heat engine PPT courseware download, Mechanical energy internal energy and its conversion PPT courseware download ,.PPT format;
For more information about the PPT courseware "Thermal Engine Mechanical Energy Internal Energy and Its Transformation", please click the Thermal Engine PPT Mechanical Energy Internal Energy and Its Transformation PPT tab.
"The Efficiency of Heat Engines" Utilization of Internal Energy PPT teaching courseware:
"The Efficiency of Heat Engines" Utilization of Internal Energy PPT Teaching Courseware Part One: A Classification Practice of Knowledge Points Knowledge Point 1 Calorific Value of Fuel 1. The ratio of the heat released when a certain fuel _________ is burned to its mass is called the calorific value of the fuel. The calorific value of gasoline is 4.6..
"The Efficiency of Heat Engines" Utilization of Internal Energy PPT Download:
"The Efficiency of Heat Engines" Utilization of Internal Energy PPT Download Part One Content: Tutorial Design Learning Point 1 Calorific Value of Fuel Information 1: In modern society, most of the energy used by humans is still obtained from the combustion of fuel. There are many types of fuels. Solid fuels include wood...
"The Efficiency of Heat Engines" Utilization of Internal Energy PPT:
"The Efficiency of Thermal Engine" Utilization of Internal Energy PPT Part One: Knowledge Points Basic Knowledge Point 1 The calorific value of fuel 1. At 22:07 on September 19, 2018, our country used the Long March 3B carrier rocket to launch an arrow at the Xichang Satellite Launch Center The thirtieth successful launch of a double star...
File Info
Update Time: 2024-12-03
This template belongs to Physics courseware Beijing Normal University Ninth Grade Physics Volume 1 industry PPT template
"Heat Engine" Mechanical energy, internal energy and their conversion PPT courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Heat Engine" Mechanical energy, internal energy and their conversion PPT courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Heat Engine" Mechanical energy, internal energy and their conversion PPT courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview