▎WumingKant/Report
On October 23 this year, Biogen and Eisai jointly announced that plans to The FDA submitted a biologics application (BLA) for the β-amyloid antibody aducanumab to treat patients with early Alzheimer's disease (AD). In March of this year, Biogen and Eisai announced that the phase 3 clinical trial of aducanumab was terminated because the independent data monitoring committee believed that it was unlikely that aducanumab would achieve the expected efficacy. As soon as the news of this "shocking reversal" came out, it can be said that "one stone caused a thousand waves", which aroused widespread heated discussions in the industry and the public. Since 2003, the U.S. FDA has not approved any new drug for AD. The "resurrection" of aducanumab has undoubtedly brought a glimmer of hope to thousands of AD patients . Bijian announced the two 3 However, these data have puzzled industry experts. Because aducanumab obtained diametrically opposite results in two phase 3 clinical trials with the same design. What caused the vastly different performance of aducanumab in the two trials? The data released by Biogen on October 23 did not give a convincing explanation. 
Today, Biogen announced at the 12th Clinical Trials on Alzheimer's Disease (CTAD) conference that everyone has been looking forward to, Detailed results of the much-anticipated aducanumab clinical trials in EMERGE and ENGAGE. In today’s article, WuXi AppTec’s content team will combine media reports and Biogen’s report PPT file to share with readers an in-depth interpretation of the detailed data of aducanumab.
What is the reason for the difference in the clinical trial results between EMERGE and ENGAGE?
Among the data released by Biogen on October 23, the most surprising thing is that EMERGE and ENGAGE, two clinical trials with the same theoretical design, are probably the most surprising. The experiment yielded completely different results. In the EMERGE clinical trial, the group of patients who received high-dose aducanumab had a 22% reduction in the CDR-SB score, a measure of cognitive performance (lower scores indicate a slower progression of disease symptoms), while In the ENGAGE clinical trial, the CDR-SB score of the patient group who also received high-dose aducanumab treatment increased by 2%. Is there any difference in the treatment received by these two groups of patients? 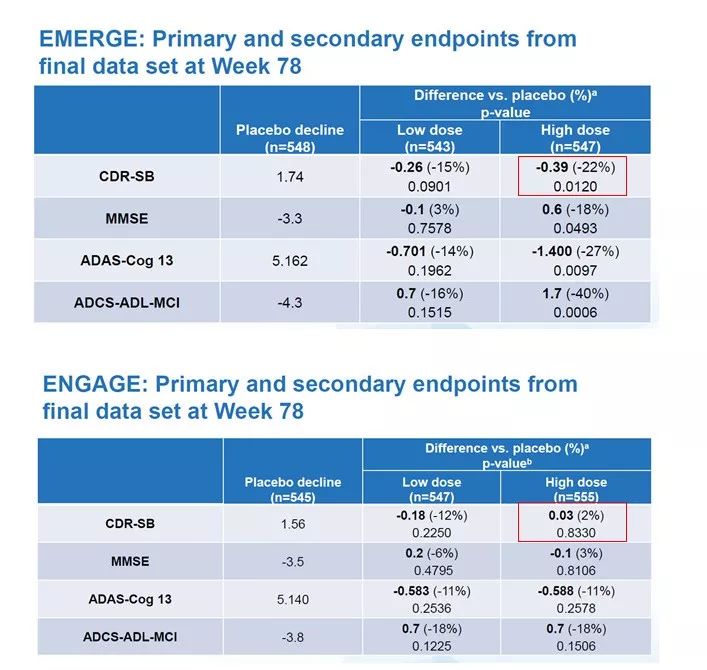
▲EMERGE and ENGAGE clinical trial primary and secondary endpoint data, the red box is the change of CDR-SB score in the high-dose group (picture source: reference [1])
This starts with the clinical design of these two trials. AD patients participating in clinical trials can be divided into two groups, one group of patients carrying the allele called ApoE ε4 (ApoE ε4+), while the other group of patients does not carry the ApoE ε4 allele ( ApoE ε4-). There are three types of alleles encoding ApoE protein in the population, namely ApoE ε4, ApoE ε3, and ApoE ε2. The group carrying ApoE ε4 has a significantly higher risk of developing AD than other groups. At the same time, Biogen's preliminary trial results showed that ApoE ε4+ patients were more likely to develop a disease called ARIA when receiving aducanumab treatment. side effects. ARIA stands for amyloid associated imaging abnormality, which can be a sign of brain edema or microbleeds. Because of this side effect, patients with ApoE ε4+ received aducanumab at a maximum dose of 6 mg/kg in the original trial design. The highest acceptable dose for patients without ApoE ε4 is 10 mg/kg. That is to say, in the same high-dose group, according to whether the patients carry ApoE ε4, the dose of aducanumab they receive is different. 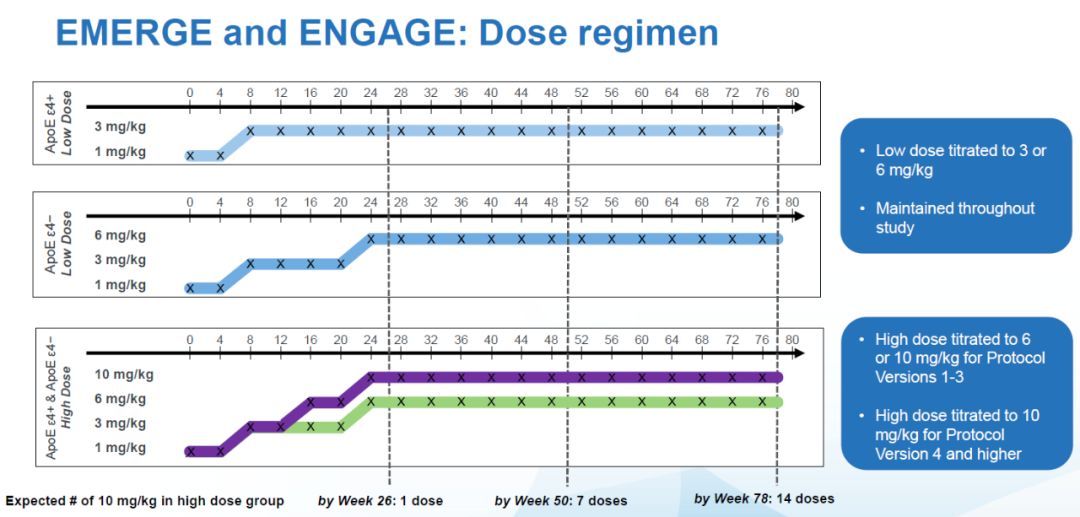
▲EMERGE and ENGAGE drug delivery process (picture source: reference [1])In March 2017, Biogen made changes to the test process, and according to the ARIA study, Biogen found that this One side effect has little impact on the patient's health, so in the updated trial procedure (Protocol Version 4, Pv4), patients with ApoE ε4 can also receive aducanumab at the highest dose of 10 mg/kg. This means that in the high-dose group, patients with ApoE ε4 did not receive a uniform dose of aducanumab, some patients may receive 6 mg/kg, while others receive is 10 mg/kg. 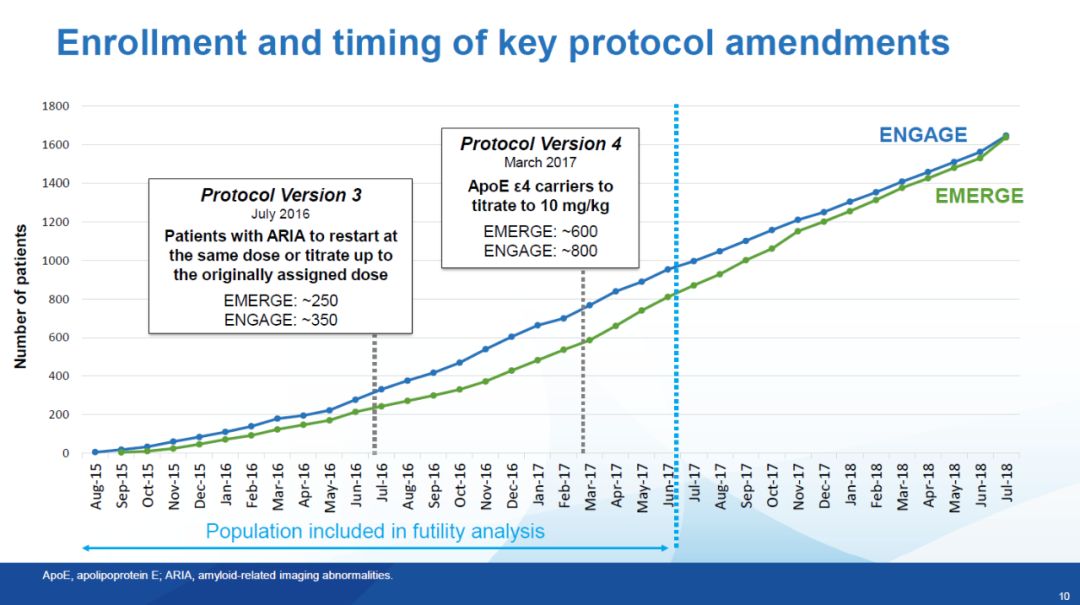
▲Bojian’s changes to the test process (picture source: reference [1])Due to the different speeds of recruiting patients in the EMERGE and ENGAGE trials, in the high-dose group, 10 mg/kg of aducanumab was actually received There was a significant difference in the proportion of patients treated. Before the trial protocol change,Only 21% of patients in the high-dose arm of EMERGE were able to receive 14 doses of 10 mg/kg. In the high-dose arm of ENGAGE, the proportion of this patient group dropped to 15%. This is an important difference between these two groups of patients. 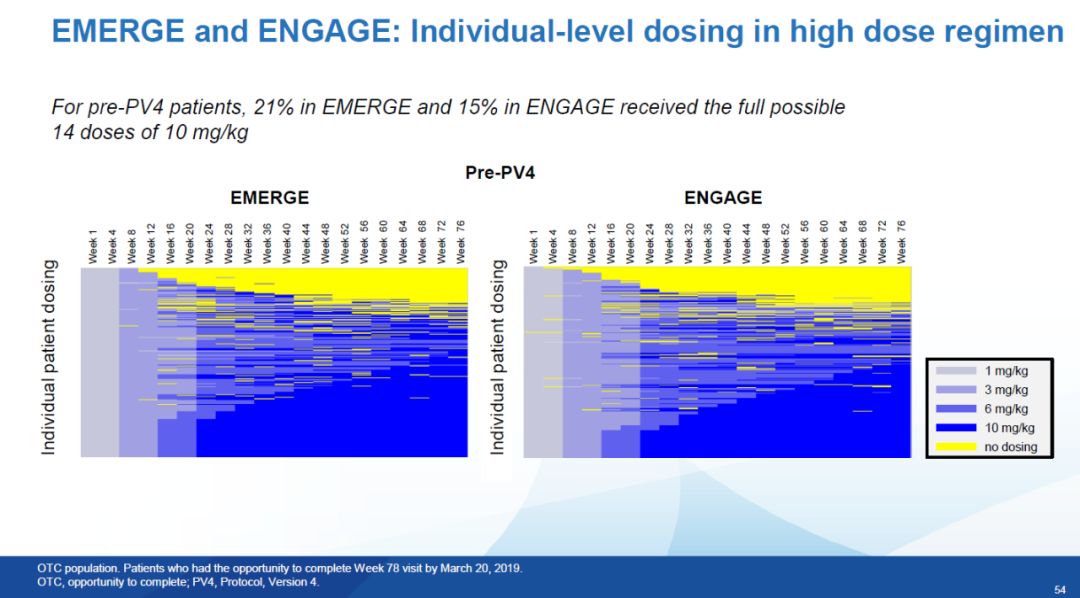
▲Before Pv4, the treatment data diagram of patients in the EMERGE and ENGAGE high-dose groups, the blue represents the injection of aducanumab at 10 mg/kg (picture source: Reference [1]) The EMERGE and ENGAGE trial data were similar in the patients enrolled under the new trial procedure
Under the new trial process (Post-PV4), the proportion of enrolled patients who can receive high-dose aducanumab at 10 mg/kg has increased significantly High, 51% of patients in the EMERGE arm were able to receive 14 doses of 10 mg/kg throughout the clinical trial, while 47% of patients in the ENGAGE arm were able to receive 14 doses of 10 mg/kg. 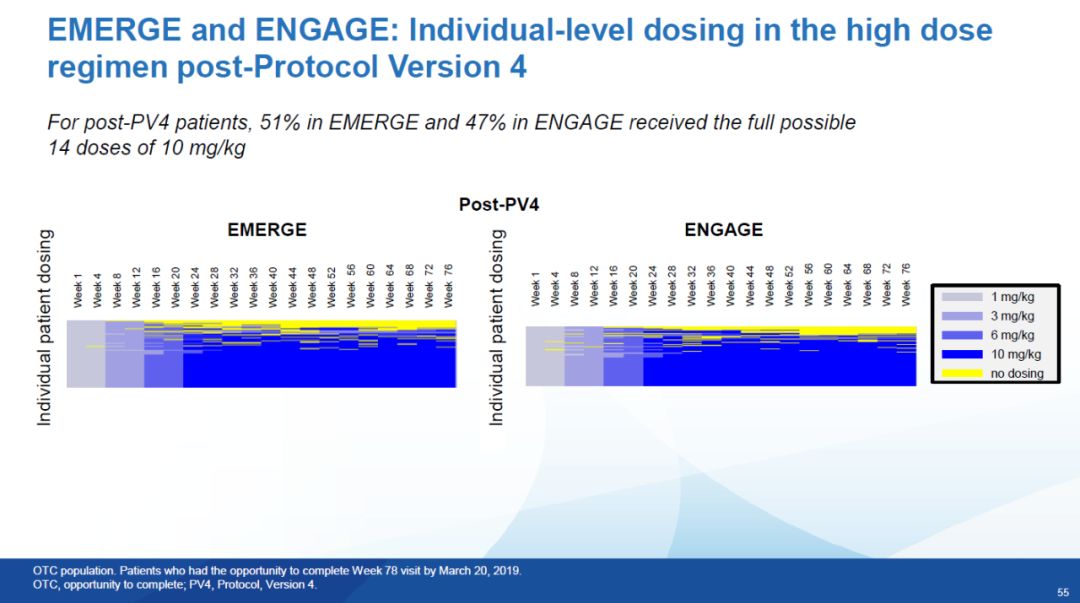
▲After Pv4, the treatment data diagram of patients in the EMERGE and ENGAGE high-dose groups, the blue represents the injection of aducanumab at 10 mg/kg (picture source: reference Data[1])
If we focus on these there are more The subgroup of patients with a high probability of receiving the highest dose of aducanumab, then the data analysis found that the data of the EMERGE and ENGAGE clinical trials are very similar. Among patients enrolled under the new protocol, CDR-SB scores were reduced by 30% and 27% in the high-dose arm of the EMERGE and ENGAGE trials, respectively. These data demonstrate that continued aducanumab at a dose of 10 mg/kg achieved consistent disease symptom relief in both the EMERGE and ENGAGE groups. 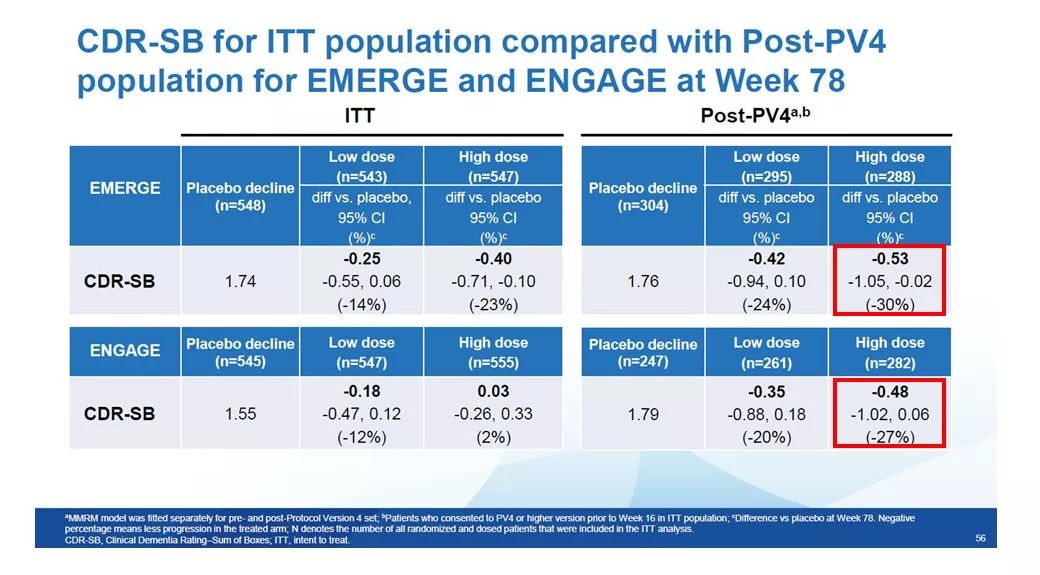
▲The improvement of CDR-SB scores in the Post-Pv4 patient population was similar in both clinical trials (Source of picture: Reference [1])
Many questions remain to be resolved
The detailed results of aducanumab show that if patients can continue to receive high-dose aducanumab 10 mg/kg, then they recognize Declines in capacity can be ameliorated. This does not mean that the patient's cognition has improved, but that the rate of decline has slowed. However, for AD patients, this has very important significance. This means that for mild patients , they have more time to take care of themselves, they are able to work, shop, travel... The biggest concern for AD patients is not being able to take care of themselves, I think this improvement is very important for our patients , much more than the change in score on the memory test.”
Biogen plans to submit to the FDA early next year Regulatory filing, meanwhile, The company will restart the discontinued Phase 3 clinical trial to restart aducanumab in patients enrolled in the EMERGE and ENAGE trials.
However, even with the release of the detailed results of aducanumab, there are still many unanswered questions about aducanumab. For example:
What is the effect of Aducanumab on AD patients who do not carry ApoE ε4?
The data released by Biogen did not include the patient’s The curative effect is classified according to whether ApoE ε4 is carried. Because the change in Pv4 is to allow patients with ApoE ε4 to use higher doses of aducanumab, it means that the positive effect of Pv4 may be due to the improvement of symptoms in ApoE ε4 positive patients . So does high-dose aducanumab have the same effect on patients who do not carry ApoE ε4? From the perspective of FDA approval, this will determine the size of the patient population for which aducanumab is applicable. Are the side effects of aducanumab affecting its approval?
ARIA is a common side effect of beta-amyloid antibody drugs in clinical trials. In detailed data published by Biogen, ARIA-associated cerebral edema (ARIA-E) occurred in ~35% of patients treated with high-dose aducanumab, and in ~18% to 22.7% of patients ARIA-associated microhemorrhages (ARIA-H) occurred. The frequency and severity of ARIA events are also of concern because the FDA will weigh its efficacy versus risk ratio when reviewing aducanumab's regulatory application. HoweverBojian said that most patients with ARIA did not show obvious symptoms, and no patient died due to ARIA. And with more clinical trials of these drugs underway, health care providers now have better control over ARIA, so it may no longer be a limiting factor in treatment. 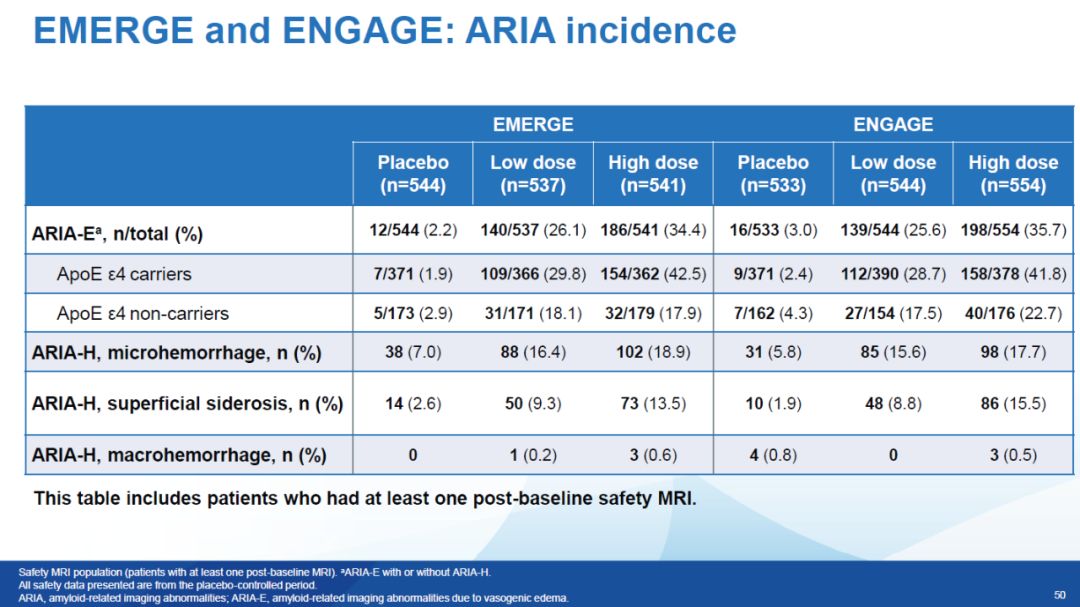
▲Aducanumab ARIA side effects information (picture source: reference [1])Does Biogen need to conduct a new clinical trial?
Subgroup analysis of patients who had the opportunity to continue to receive 10 mg/kg dose of aducanumab treatment showed consistent results in the EMERGE and ENGAGE clinical trials Efficacy, but this was a post hoc analysis of a subgroup of the trial. Brian Skorney, an analyst at Baird, said, "We believe this analysis should be viewed as a hypothesis-generating exploration.To really be sure (high-dose aducanumab treatment) Biogen needs to conduct a new prospective clinical trial.”
In this regard, Biogen stated that the The company will respect the science and follow FDA's review mechanisms and decisions. However, it may take 3-5 years to conduct another clinical trial, which will be a long wait for patients who are eagerly expecting new drugs to improve disease symptoms. It is true that no amount of detailed data can answer the most important question in everyone’s mind, which is this Whether the drug under investigation can be approved by the FDA for marketing. Biogen said that submitting the regulatory application as soon as possible will be the company's top priority. Let us wait and see what the prospect of aducanumab is.
Click "Read More" at the end of the article You can download the PPT of Biogen reporting the detailed results of aducanumab. 
References:
[1] EMERGE and ENGAGE Topline Results: Two Phase 3 Studies to Evaluate Aducanumab in Patients With Early Alzheimer's Disease. Retrieved December 5, 2019. From http://investors.biogen. com/static-files/ddd45672-9c7e-4c99-8a06-3b557697c06f
[2] Biogen Plans Regulatory Filing for aducanumab in Early Alzheimer's Disease. Retrieved December 4, 2019, from https://biogenalzheimers.com/
[3] BIOGEN PLANS REGULATORY FILING FOR ADUCANUMAB IN ALZHEIMER'S DISEASE BASED ON NEW ANALYSIS OF LARGER DATASET FROM PHASE 3 STUDIES. Retrieved December 4, 2019, from http://investors.biogen.com/news-releases/news-release ase-details /biogen-plans-regulatory-filing-aducanumab-alzheimers-disease
[4] 4 questions key to making sense of new data on Biogen's rescued Alzheimer's drug. Retrieved December 4, 2019, from https://www.statnews. com/2019/12/04/4-questions-key-to-making-sense-of-new-data-on-biogens-resurrected-alzheimers-drug/
[5] Aducanumab Update. Retrieved December 4, 2019 , from http://investors.biogen.com/static-files/5a31a1e3-4fbb-4165-921a-f0ccb1d64b65
[6] Skeptics pounce as Biogen details positive subgroup analysis on aducanumab — and both sides are digging in. Retrieved December 5, 2019, from https://endpts.com/skeptics-pounce-as-biogen-details-positive-subgroup-analysis-on-aducanumab-and-both-sides-are-digging-in/
[ 7] Detailed data on Biogen's recovered Alzheimer's drug raise more questions than answers. Retrieved December 5, 2019, from https://www.statnews.com/2019/12/05/detailed-data-on-biogens-resurrected-alzheimers- drug-raise-more-questions-than-answers/
Copyright statement: This article is from the content team of WuXi AppTec. Individuals are welcome to repost it to Moments. Media or organizations are not allowed to repost it on other platforms in any form without authorization. For reprint authorization, please reply "reprint" on the "WuXi AppTec" WeChat official account to obtain reprint instructions.
Articles are uploaded by users and are for non-commercial browsing only. Posted by: Lomu, please indicate the source: https://www.daogebangong.com/en/articles/detail/Indepth%20interpretation%20How%20to%20understand%20the%20detailed%20data%20of%20the%20new%20Alzheimers%20disease%20drug%20that%20returns%20to%20life.html










 支付宝扫一扫
支付宝扫一扫 
评论列表(196条)
测试