【Abstract】Teaching plans are an indispensable teaching skill for teachers. Teacher qualification certificate interviews and test preparation need to write teaching plans. The editor specially compiled them The teaching plan for the interview of junior high school chemistry teacher qualification certificate - "burning and extinguishing fire", I hope it can help the candidates!
1. Requirements for teaching purposes
Basic knowledge and skills:
Recognize the conditions under which combustion occurs and know Simple principles and methods of fire extinguishing.
Process and method:
1. By studying the conditions of combustion , know the method of exploring the problem;
2. Use experimental learning to compare in role in chemistry learning;
3. Through activities and exploration, Experience the scientific method of drawing conclusions by analyzing the obtained facts using methods such as comparison and induction.
Emotional attitudes and values:
1. Know the essence of combustion and its have a major impact on life.
2. Understand fire hazards and protection Methods, have the awareness of safe use of fire, learn simple fire fighting skills and escape methods.
3. Make students realize: Only when man masters the laws of nature can he make better use of and transform nature.
2. Teaching key burning conditions fire extinguishing Principle
3. Teaching method: Experimental exploration , multimedia assistance, group cooperation, exchange and discussion.
4. Teaching process:
Introduction: Classmates, speaking of What do you immediately associate with burning? (Answer: fire) So what kind of impact does fire have on our lives? Please watch a video. (Show Video 1)
Teacher: Students, read it What do you think about this video? Please express your opinion.
(Students express their opinions, pay attention to encourage students)
Teacher: Since burning is important to us Life has such a big impact, students, in this class, we will explore the issues related to combustion and fire extinguishing, so that we can make good use of the fire produced by combustion to serve us human beings.
Teacher: See you, students After a lot of burning scenes, what characteristics do you find in ordinary burning?
Health: glow, heat release, etc.
Teacher: Burning is generally combustible React with what substance?
Health: Oxygen
Teacher: Yes, burning is like What you said is a luminous, exothermic violent oxidation reaction.
How to make the combustion go smoothly Carry out? What conditions are required for combustion? We might as well start with the following questions:
1. All substances can Burning?
Given some substance, the choice can Burning:
wooden sticks, candles, small coals blocks, pebbles, paper, alcohol, glass, cotton
2. Combustible substances are in Can it burn under what conditions?
Explore one:
Minimum temperature required for the substance to burn Also called the ignition point of the substance.
Students experiment and explore, and then communicate Discuss and analyze the three conditions of combustion: writing on the blackboard: combustibles, oxygen, and temperature reaching the ignition point.
These three conditions need to be at the same time Is it possible to have one or only one of them? Case analysis proves.
How to explain the following phenomenon?< /span>
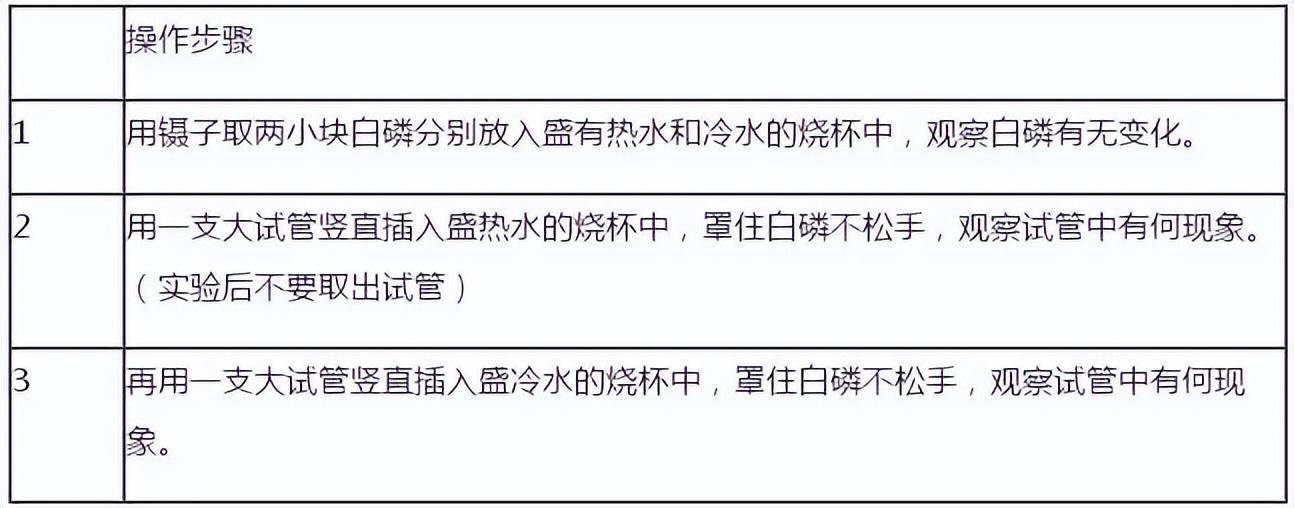
Demo: hold a small amount of Put the large test tubes of red phosphorus and white phosphorus in hot water at the same time, and observe the phenomenon.
Please describe the experimental phenomenon and analyze reason. Explain that different substances have different ignition points.
The table below shows some Flash points of common substances:

Generally speaking, those with low ignition point Substances are more flammable, and substances with a higher ignition point are more difficult to ignite.
Transitional Quote: We Know Burning Three conditions must be met at the same time, so what should we do if we need to extinguish the fire? (Answer: Destroy the burning conditions!) How to destroy? We might as well take the incidents around us as an example to think of a way.
Teacher: Please think about it, students What should I do in the following situations? (Students are required to tell the method and the principle of its application, reflecting the new concept of entering chemistry from life and using chemical knowledge to solve practical problems.)
The fuse of a pack of dynamite is far away is lit!
The frying pan caught fire at home, why? Do?
The cardboard boxes stacked with sundries caught fire , what should I do?
Students thinking, summarizing: basis Combustion conditions lead to the principle of fire extinguishing: destroying one of the conditions required for combustion can achieve the purpose of fire extinguishing, and can be taken: remove or isolate combustibles; isolate air or oxygen; reduce the temperature below the ignition point of combustibles.
Discussion: In case of fire, What measures should you take? (principle: small self-help, big escape)
Let students think and discuss (students There are various and even wrong plans. But it doesn’t matter, teachers and students can discuss and judge different plans, reach a consensus, and come up with a more scientific plan.)
Multimedia playback courseware: fire scene escape animation.
Articles are uploaded by users and are for non-commercial browsing only. Posted by: Lomu, please indicate the source: https://www.daogebangong.com/en/articles/detail/Classic%20Lesson%20Plan%20Junior%20High%20School%20Chemistry%20Burning%20and%20Extinguishing%20Fire.html

 支付宝扫一扫
支付宝扫一扫 
评论列表(196条)
测试